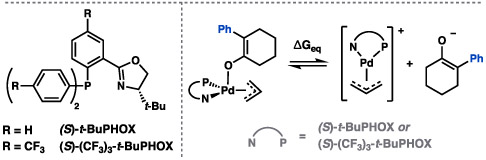
Table 2.
Effects of Solvation and Ligand Electronics on Enantioselectivity
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Solvent [ε] | ee (%)a | Yield 22 (%)b | ΔGeqc |
| 1 | (S)-t-BuPHOX | THF [7.4] | 23 | 99 | 1.8 |
| 2 | (S)-t-BuPHOX | PhMe [2.4] | 28 | 96 | 24.0 |
| 3 | (S)-(CF3)3-t-BuPHOX | PhMe [2.4] | 32 | 90 | 31.0 |
| 4 | (S)-(CF3)3-t-BuPHOX | 2:1 MeCy/PhMe [2.1] | 36 | 69d | 35.2 |
 | |||||
Obtained by chiral SFC.
Determined by 1H NMR with respect to internal standard.
Change in free energy (in kcal/mol, 1 M standard state) from square pyramidal enolate to free ions.
34% 20 remaining after 12 h (1H NMR with respect to internal standard).
