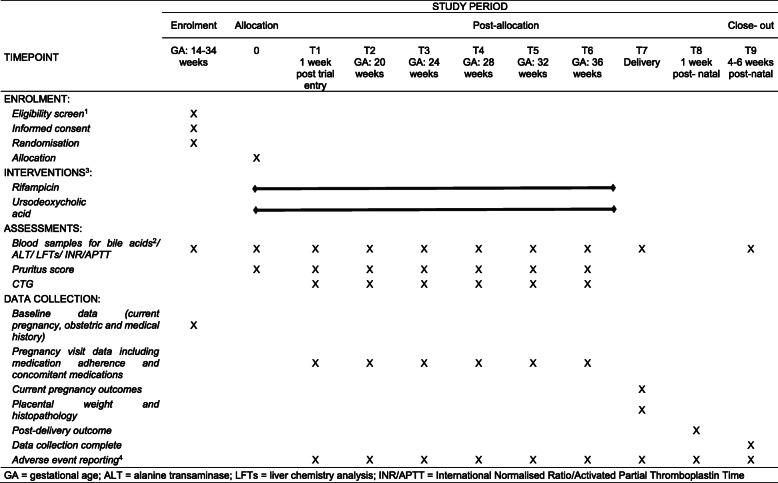
Table 1.
Trial Timeline
GA gestational age, ALT alanine transaminase, LFTs liver chemistry analysis, INR/APTT International Normalised Ratio/Activated Partial Thromboplastin Time
Notes to Table 1
1All screening assessments are part of normal clinical practice
2Bile acid samples need to be drawn shortly before a scheduled dose of UDCA, though fasting is not required. Women previously prescribed UDCA for treatment of mild ICP, and agreeing to randomisation, will have basal assessments performed 4–7 days after ceasing such therapy prior to randomisation into the trial
3Study treatment started after randomisation. Dose to be adjusted by PI if indicated by symptoms and/or blood tests taken during normal clinical practice
4Adverse events will be recorded from study entry until post-delivery discharge of woman and baby. All SAEs are to be reported to the Coordinating Centre within 24 h of Investigator knowledge