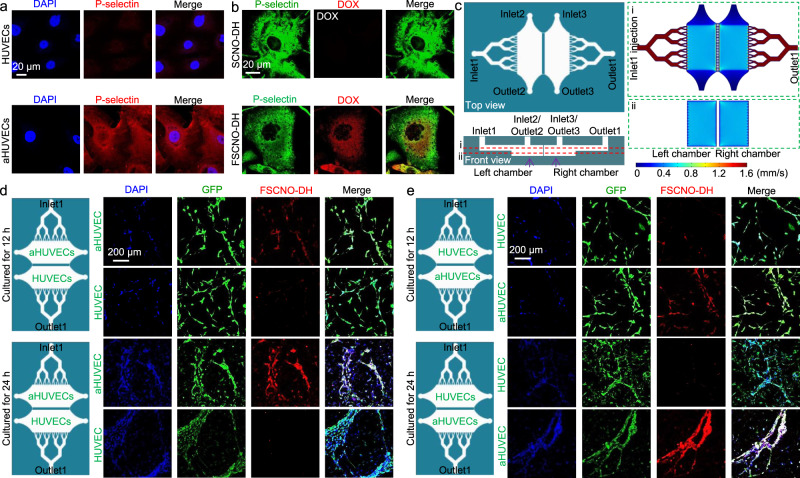
Fig. 5. P-selectin targeting capability of the FSCNO nanoparticles under both static and dynamic/microfluidic cultures.
a Confocal images showing the expression of P-selectin in activated human umbilical vein endothelial cells (aHUVECs) but not in HUVECs without activation. b Confocal images of precooled aHUVECs after incubated with SCNO-DH and FSCNO-DH nanoparticles (10 μg ml−1 for DOX and 5 μg ml−1 for HM). The cells were precooled in ice for 3 h to stop its metabolic and uptake activity. c Top and side views of the microfluidic device for studying the P-selectin targeting capability of the FSCNO-DH nanoparticles under dynamic culture condition. Detached cells can be injected into device through inlet2 or inlet3 and cultured in the chambers. Medium containing nanoparticles can be injected through inlet1 and flows out the device via outlet1. Also shown are the computational modeling results of velocity distribution at the middle planes of the channels and chambers, in the top and bottom PDMS parts as indicated by the red dashed lines i and ii, respectively. The data show that the velocity of injected nanoparticle solution (inlet1) is nearly homogeneous in the two chambers. d, e Scheme and confocal images of green fluorescence protein (GFP)-expression HUVECs (in the chamber next to outlet1 for d and in the chamber next to inlet1 for e) and aHUVECs (in the chamber next to inlet1 for d and in the chamber next to outlet1 for e) cultured in the microfluidic device for 12 and 24 h after binding with FSCNO-DH nanoparticles in the perfusion medium for 3 h. The data indicate that the FSCNO-DH nanoparticles can target the aHUVECs efficiently regardless of the culture conditions (i.e., static versus dynamic, and for the latter: close or away from the inlet1).