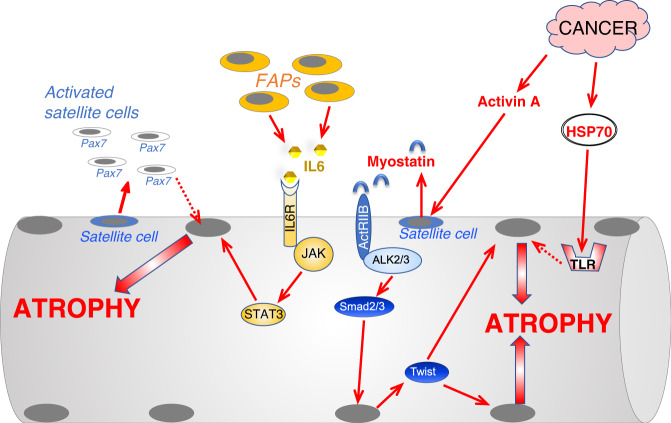
Fig. 5. Scheme of the principal pathways induced by the interstitial cells or by cancer that control protein degradation and muscle atrophy.
Cancer growth induces hyperproliferation of Pax7-positive muscle stem cells, named satellite cells (SC), that do not fuse with myofiber and triggers an atrophy program whose insights are still unclear. When exposed to an increased TGFβ signaling, satellite cells express Twist that induces myostatin expression and secretion. The release of myostatin causes a second wave in myofibers where Twist and Smad3 cooperate to promote muscle atrophy. Denervation, cause expansion of the fibro-adipogenic precursor (FAP) cells which induce an inflammatory response via IL6 leading to Stat3 activation in muscle and induction of an atrophy program. Cancer derived exosomes deliver HSP70/90 to myofibers that activate TLR4 and an atrophy program. Dotted lines depict pathways whose molecular mechanisms and role in adult skeletal muscle have yet to be completely defined.