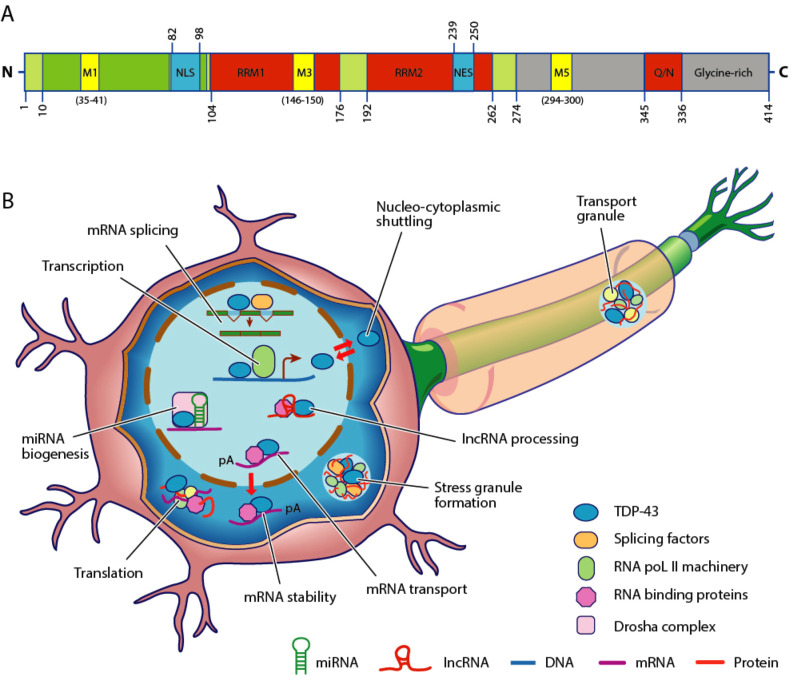
Figure 1.
(A) Structure of TAR DNA-binding protein 43 (TDP-43) protein. The TDP-43 protein contains 414 amino acids and is comprised of an N-terminal region with a nuclear localisation signal (NLS). In addition, the protein consists of two RNA recognition motifs (RRM1 and RRM2), a nuclear export signal (NES) and a C-terminal domain with a glutamine/asparagine-rich (Q/N) and glycine-rich regions. Mitochondrial localisation motifs (M1; M3; M5) are also evident. Pathogenic mutations are predominantly located within the C-terminal region which can exhibit prion-like properties. The numbers represent amino acid lengths. (B) The TDP-43 protein is critical for mediating RNA metabolism. In the nucleus, TDP-43 is important for transcription and splicing of messenger RNA (mRNA), as well as maintaining RNA stability (pA) and transport to nucleus. In addition, TDP-43 regulates biogenesis of microRNA (miRNA) and processing of long non-coding RNA (lncRNA). Although predominantly located within the nucleus, TDP-43 shuttles between the nucleus and the cytoplasm. In the cytoplasm, TDP-43 participates in mRNA stability, translation, formation of stress and ribonucleoprotein (RNP) transport granules.