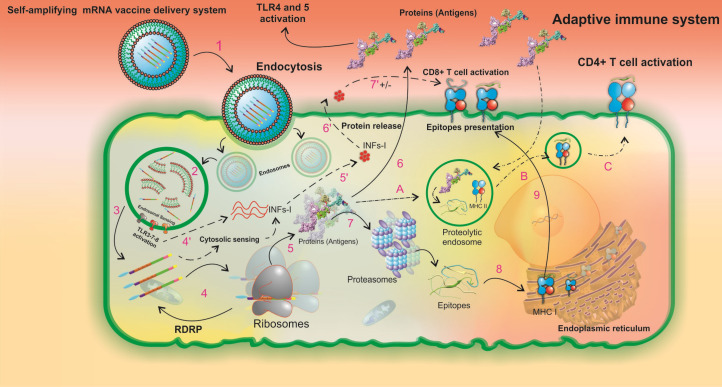
Fig. 10.
A schematic illustration of the intracellular processing of LNPs formulated the SAM vaccine and the subsequent innate and pathogen-specific immune responses. The in vitro transcribed SAM vaccine is formulated as a targeted vaccine delivery system (VDS), which is internalized by the antigen-presenting cells through receptor-mediated endocytosis (1). The targeted VDS is escaped from the endosomal compartment, and the initial endosomal RNA sensing by TLRs (mainly TLRs 3, 7, and 8) is activated (2). Upon SAMV endosomal escape, two main pathways of innate and adaptive immune responses can be activated (3). In the innate immune responses, steps 4' to 7' can occur. Both the SAM vaccine construct and the initial endosomal RNA sensing system activate the secondary RNA sensing system which is induced by cytosolic pathogen recognition receptors and then results in the production of type I interferons (INFα/β) (4', 5'). INFs are secreted (6'). The regulatory impacts of INFs are imposed on T-cell activity pathways (7'). In the Adaptive immune responses,steps 4-9 can occur. The in vivo translation of SAMV construct, the formation of RNA-dependent RNA polymerase (RdRp) complex, and the beginning a self-replication machinery for enhancement of the protein yield occur (4). The newly produced recombinant proteins have three possible destinies (5). First, protein is released to the extracellular space and its TLR agonists (i.e. RS09, and FlgC) can activate both TLRs 4 and 5, respectively (6). Second, protein is degraded by proteasomes to the small peptide fragments (7). The peptide fragments are processed by the endoplasmic reticulum (8). The MHC class I-epitope complexes are presented on the cell surface (9). Third, the peptides enter the proteolytic endosomes (A) to form the MHC class II-epitope complexes (B) and to be presented on the cell surface (C). LNP: lipid-nanoparticle. SAMV: self-amplifying mRNA vaccine.