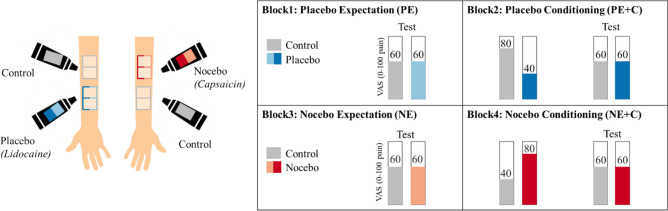
Figure 4.
Placebo and nocebo paradigm. (A) Ointments and marked skin areas. For experimental block 1 and 2, a placebo ointment (“Lidocaine”) and a control ointment were introduced. It was explained that “Lidocaine” would exert an analgesic effect. Four skin areas were marked on one forearm of the participants. The skin areas were color-coded for “Lidocaine” and control treatment. For experimental block 1 and the conditioning part of experimental block 2, one control area and one placebo area were used, whereas the second phase (“test”) in experimental block 2 was performed on the remaining two skin areas on the same forearm. For experimental block 3 and 4, the nocebo ointment (“Capsaicin”) was introduced to the participants, as a treatment with a hyperalgesic effect. Four skin areas were marked on the other forearm. For experimental block 3 and the conditioning part experimental block 4, one control area and one nocebo area were used. Afterwards, the second phase (“test”) of experimental block 4 was performed at another nocebo area and control area. The order of the left and right arm and the position of placebo, nocebo and control were counterbalanced across participants. (B) Placebo and nocebo paradigm. In experimental block 1, placebo expectation (PE) effects were tested: Participants were exposed to two times eight heat stimuli, which were calibrated to match 60 on a 0 to 100 VAS (VAS60). In experimental block 2, placebo conditioning (PC) effects were tested. Therefore, participants were first conditioned (VAS80 stimuli for control skin area and VAS40 for placebo skin area) on the previous skin areas, and then tested again with VAS60 on the remaining two skin areas. Afterwards participants were introduced to the nocebo treatment and experimental block 3 and 4 were performed in the same manner as experimental block 1 and 2, except that the nocebo skin area was conditioned with VAS80 and the control skin area was conditioned with VAS40, respectively. The overall order of the blocks was fixed for all participants, while the order of treatment (placebo or nocebo) and control was counterbalanced across participants.