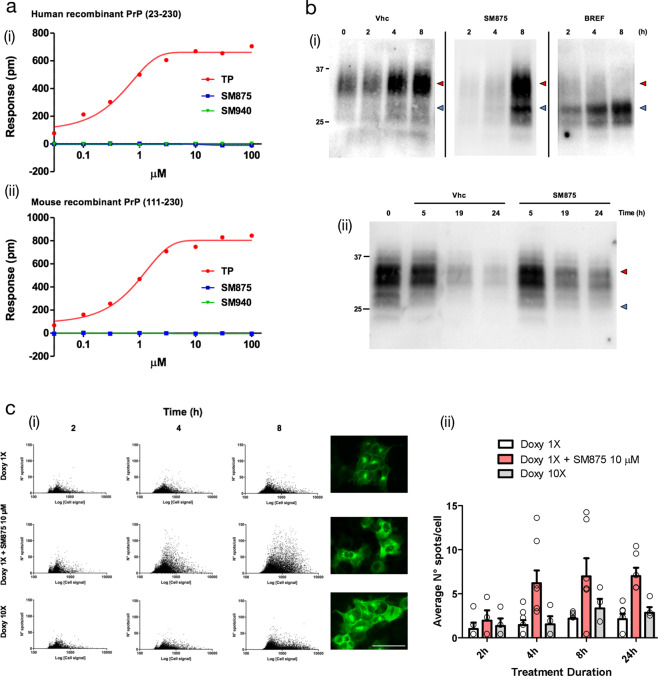
Fig. 5. SM875 acts exclusively on non-native, newly synthesized PrP.
a DMR was employed to assess whether SM875 has an affinity for the native conformation of PrP. Different concentrations (0.03–100 μM) of SM875, SM940 or PrP ligand Fe3+-TMPyP (TP), used as a control, were added to label-free microplate well surfaces on which either (i) full-length, human recombinant PrP (23–230) or (ii) N-terminally deleted mouse recombinant PrP (111–230) had previously been immobilized. All signals were fitted (continuous lines), when possible, to a sigmoidal function using a 4PL non-linear regression model. In contrast to SM875 and SM940, TP shows a detectable affinity for both full-length, human, and N-terminally deleted, mouse PrP molecules (for full-length PrP, Kd = 0.67 ± 0.05, R2 = 0.99). b In order to dissect the effect of SM875 on nascent vs mature, native PrP molecules, we turned to RK13 cells expressing mouse PrP under control a doxycycline-inducible promoter. (i) PrP expression was induced over 8 h, in the presence of SM875 (10 μM), brefeldin-1A (BREF, 10 μM), or vehicle (Vhc) control, samples were collected at different time points (indicated) and PrP signals were visualized by western blotting. Signals were detected by probing membrane blots with anti-PrP antibody (D18). As expected, in control cells, the level of full-length PrP (red arrowheads) increases in a time-dependent fashion. Conversely, a lower molecular weight band (blue arrowheads) is detected in brefeldin-treated cells. (ii) Next, we designed an experiment to test the effect of SM875 exclusively on mature, natively folded PrP. PrP expression was induced for 24 h, in the absence of any additional treatment. Doxycycline was then removed, and after 4 h without inducer, the cells were exposed to SM875 or Vhc control, and subsequently lysed at different time points (indicated). In this experimental setting, cells are exposed to SM875 only when all PrP molecules are synthesized and likely in transit to, or already reached the plasma membrane. In these conditions, normal PrP patterns appear in both compound-treated and Vhc-treated cells. c A high-content approach was employed to the same experimental setting described above by analyzing the localization of PrP after immunostaining with an anti-PrP antibody (D18) coupled to an Alexa 488 secondary antibody. (i) The expression of PrP was induced for 2, 4, or 8 h by doxycycline 1× (0.01 mg/mL) or 10× (0.1 mg/mL) and the effect of SM875 (10 μM) incubated with doxycycline 1× was measured by Harmony software after the image acquisition performed by Operetta Imaging System. Green spots detected in cells were quantified and plotted against the total green fluorescence relative to each cell. Representative images were acquired at 8 h of incubation, scale bar 50 μm. (ii) Quantification of the average number of green spots per cell in wells incubated for 2, 4, 8, and 24 h with doxycycline 1× (white bars), doxycycline 10× (gray bars) and doxycycline 1× + SM875 (red bars). Quantification of at least three independent experiments.