Figure 2.
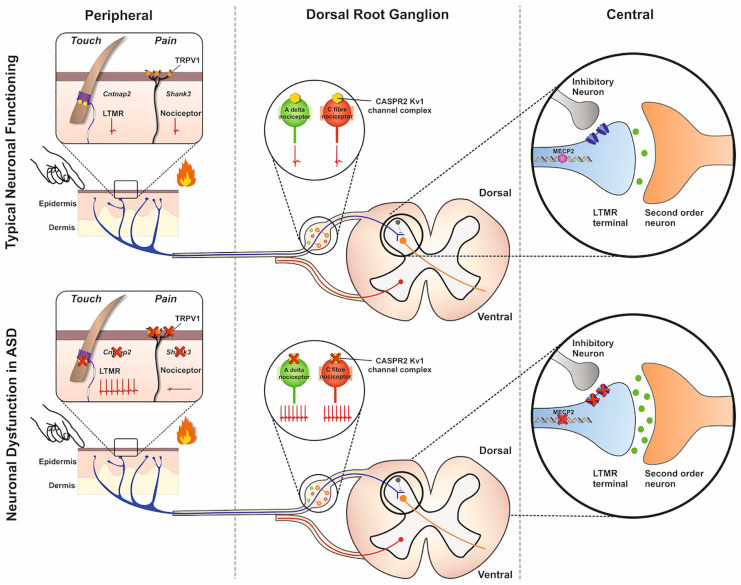
Dysfunction of primary sensory neurons in autism spectrum disorders (ASD). By genetically altering ASD-linked genes, several mouse models have been developed. Some of these models have shown phenotypic changes in somatosensation associated with primary sensory neuron dysfunction. This dysfunction is linked not only to the synapse (the central terminal of the dorsal horn of the spinal cord) of these neurons but also to other neuronal compartments (e.g., the peripheral terminal in the skin and the cell soma). Loss of Cntnap2 leads to hyperexcitability in d-hairs, a type of low threshold mechanoreceptor (LTMR), due to loss of Kv1 channel function. Loss of Shank3 reduces the functional expression of TRPV1, a transduction channel important in heat hyperalgesia, in nociceptors. Soma loss of Cntnap2 also impacts onto nociceptor function resulting in hyperexcitablility of Aδ and C fibers. At the level of the synapse, loss of MECP2 results in the down regulation of GABA receptors and loss of presynaptic inhibition on LTMRs leading to increased sensitivity to tactile stimuli.