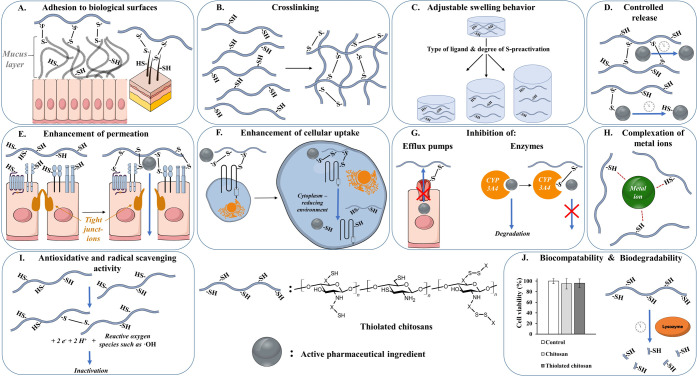
Figure 2.
Properties gained by the covalent attachment of thiol groups to chitosan. (A) Adhesion of thiolated chitosans to biological surfaces such as mucins or keratins. (B) Cross-linking of thiolated chitosans due to disulfide formation, improving in situ gelling properties and mechanical stability. (C) Adjustable swelling behavior using different ligands and degrees of S-preactivation. (D) Controlled release of covalently bound active pharmaceutical ingredients (APIs) or prolonged API release out of cross-linked polymers. (E) Enhanced API permeation due to opened tight junctions caused by the interaction of thiolated chitosans with cysteine-bearing membrane receptors and enzymes. (F) Increased absorptive endocytosis of API-loaded thiolated chitosan carriers by disulfide formation with exofacial thiols of transmembrane proteins. (G) Inhibition of efflux pumps and enzymes due to the formation of disulfide bonds with thiolated chitosans. (H) Complexation of metal ions by sulfhydryl groups of thiolated chitosans. (I) Disulfide formation of thiolated chitosans, causing inactivation of reactive oxygen species. (J) Proven biocompatibility of thiolated chitosans in comparison to unmodified chitosan and customizable degradation rate of the thiolated polymer utilizing different ligands.