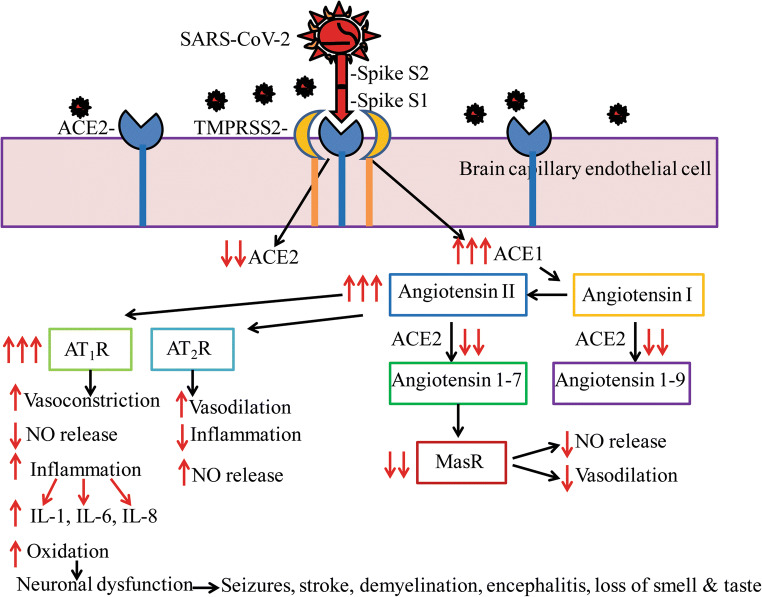
Fig. 2.
SARS-CoV-2-mediated neurodegeneration may be due to the downregulation of ACE2-mediated signaling and concomitant increase in ACE1-mediated neuroinflammation. SARS-CoV-2 binds ACE2 through the receptor-binding domain (RBD) of spike protein S1 facilitated by protease TMPRSS2, resulting in reduced functional ACE2 expression which in turn enhances ACE1 signaling including increased conversion of angiotensin I to angiotensin II. ACE2 is responsible for the conversion of angiotensin I into angiotensin 1-9 which increases nitric oxide (NO) generation and vasodilation. With reduced ACE2, angiotensin 1-9 levels are reduced and therefore NO generation is also reduced. ACE2 is also responsible for the conversion of angiotensin II into angiotensin 1-7 which enhances Mas receptor (MasR) signaling to increase vasodilation and to prevent fibrosis. With the reduction in ACE2, MasR signaling is also reduced. Increased angiotensin II levels due to decreased ACE2 activity can also enhance signaling through the type-1a (AT1R) or type-2 (AT2R) angiotensin receptors. Activation of AT2R is normally neuroprotective. However, increased AT1R signaling leads to reduced NO generation, and therefore vasoconstriction, AT1R signaling also increases oxidative stress and neuroinflammation with the overproduction of interleukins such as IL-1, IL-6, IL-8, and IL-29, all of which were confirmed in COVID-19 patients. Increased neuroinflammation in turn can cause neurodegeneration, brain dysfunction, and a variety of neurological issues as seen among COVID-19 patients. A schematic representation of a signal transduction mechanism is shown for endothelial cells of brain capillaries, and a similar mechanism is also expected in neurons and glia which also has been shown to express ACE2