Abstract
Background
Data about obstetric complications of maternal infection by SARS-CoV-2 remain sparse.
Case
A 40-year-old pregnant woman, gravida 3 para 1 with no previous obstetric complications, presented a late miscarriage at 16 weeks of gestation on day 9 of COVID-19 disease. The results of her nasopharyngeal swab for SARS-CoV-2, tested the same day, were negative, but the placenta was infected by SARS-CoV-2 and serology was positive 11 days later. No other obstetric or infectious cause was found to explain this outcome.
Conclusion
This case strongly suggests that SARS-CoV-2 may lead to a late miscarriage.
Keywords: SARS-CoV-2, Late miscarriage, Placental colonization, Obstetric outcomes
Introduction
On March 11th, 2020, the World Health Organization (WHO) declared that the outbreak of coronavirus pneumonia 2019 (COVID-19), caused by a new strain of coronavirus called SARS-CoV-2, had become a pandemic. This novel coronavirus causes viral pneumonia and mainly respiratory complications. Data about the obstetric outcomes of pregnant women infected by COVID-19 were gradually described and reported maternal and neonatal complications in infected patients [[1], [2], [3], [4], [5]].
COVID-19 infection is associated with maternal inflammation and even, in some cases with a « cytokine storm syndrome » [6]. During pregnancy, maternal infection and inflammation can induce spontaneous preterm birth and late miscarriages [[7], [8], [9]].
Obstetric complications attributable to SARS-CoV-2 have not been clearly described yet, and only few cases of fetal loss at the second trimester have been linked to COVID-19 [10,11].
We report a case of COVID-19 infection associated with a late miscarriage at 16 weeks of gestation with a placental colonization by SARS-CoV-2.
Case
At 16 weeks and 4 days of gestation, a 40-year-old woman (gravida 3, para 1) with no particular medical or surgical history was admitted to the delivery unit with painful uterine contractions and preterm premature rupture of the membranes (pPROM). Her obstetric history included an uncomplicated term vaginal delivery and an early miscarriage treated medically at six weeks of gestation. Her body mass index was 20 kg/m² and she had no addiction such as smoking. The current spontaneous pregnancy was uneventful until 15 weeks, with a normal first-trimester ultrasound and normal serum markers. The pPROM occurred 9 days after a video visit on March 21st, 2020 with her primary care physician. He considered her as a COVID-19 infected patient, based on symptoms that included a fever of 39 °C, dry cough, myalgia, and headache. He decided not to perform a SARS-CoV-2 real-time polymerase chain reaction (RT-PCR).
At admission, on March 30th, her respiratory frequency was normal at 18 breaths per minute, her ambient oxygen saturation 98 %, her blood pressure 120/82 mm Hg, heart rate 97 beats per minute, and her temperature 37.1 C degrees. Examination found the fetus expelled into the vagina, without cardiac activity. Intrauterine aspiration for complete placenta retention took place under surgical asepsis in the operating room. The aftermath was uneventful, and she was discharged the next day with no subsequent obstetric or COVID-19 complications.
Extensive laboratory tests were normal except for lymphopenia. The nasopharyngeal RT-PCR performed on March 30th was negative but SARS-CoV-2 RT-PCR of two different placental samples taken the same day were strongly positive (RT-PCR cycle threshold: 11, technique Bosphore 2019 nCoV detection kit [Anatolia geneworks]), and her SARS-CoV-2 serology on April 9th was also positive with the Covid-Presto IgG/IgM rapid test (AAZ) and confirmed by automated IgG serology on Alinity-i (Abbott) with an index of 6.4 (positivity threshold 1.4) (Table 1 ).
Table 1.
Laboratory tests results.
| Variable | Reference Range | Result |
|---|---|---|
| Blood tests (03/30/2020) | ||
| Hemoglobin (g/dL) | 12–16 | 13 |
| Hematocrit (%) | 35–47 | 37.2 |
| Platelet count (per mm³) | 150,000–400,000 | 251,000 |
| White-cell count (per mm³) | 4000–10000 | 10340 |
| Absolute neutrophil count (per mm³) | 1500– 7000 | 8 890 |
| Absolute lymphocyte count (per mm³) | 1500–4000 | 1030 |
| Prothrombin ratio (%) | > 70 | 100 |
| Activated partial thromboplastin time ratio (second) | 0.80–1.20 | 0.91 |
| Haptoglobin (g/L) | 0.55–1.46 | 2.82 |
| Sodium (mmol/liter) | 136–144 | 138 |
| Potassium (mmol/liter) | 3.5–5.0 | 4.1 |
| Creatinine (mg/dL) | 45–80 | 51 |
| Aspartate aminotransferase (U/liter) | < 32 | 36 |
| Alanine aminotransferase (U/liter) | < 32 | 47 |
| Total bilirubin (mg/dl) | < 17 | 5 |
| Kleihauer Betke test | – | 0 |
| Blood cultures | – | Negative |
| Bacteriology (03/30/2020) | ||
| Vaginal bacteriology | – | Negative |
| Urinary cytobacteriology | – | Negative |
| Placental bacteriology | – | Negative |
| Virology | ||
| Nasopharyngeal RT- PCR SARS-CoV-2 (03/30/2020) a | – | Negative |
| Nasopharyngeal RT-PCR for other respiratory viruses | – | Negative |
| Stool RT-PCR SARS-CoV-2 (04/09/2020) b | – | Negative |
| Placental RT-PCR SARS-CoV-2 (03/30/2020) b | – | Positive |
| Fetal liver and lung RT-PCR SARS-CoV-2 (03/30/2020) b | – | Negative |
| Blood serology for SARS-CoV-2 (04/09/2020) c | – | Positive |
Performed by Allplex 2019-nCoV RT-PCR Assay (Seegene).
Performed by Bosphore 2019 nCoV RT-PCR detection kit (Anatolia geneworks).
Performed by Covid-Presto IgG/IgM rapid test (AAZ) and SARS-CoV-2 IgG Alinity-i (Abbott).
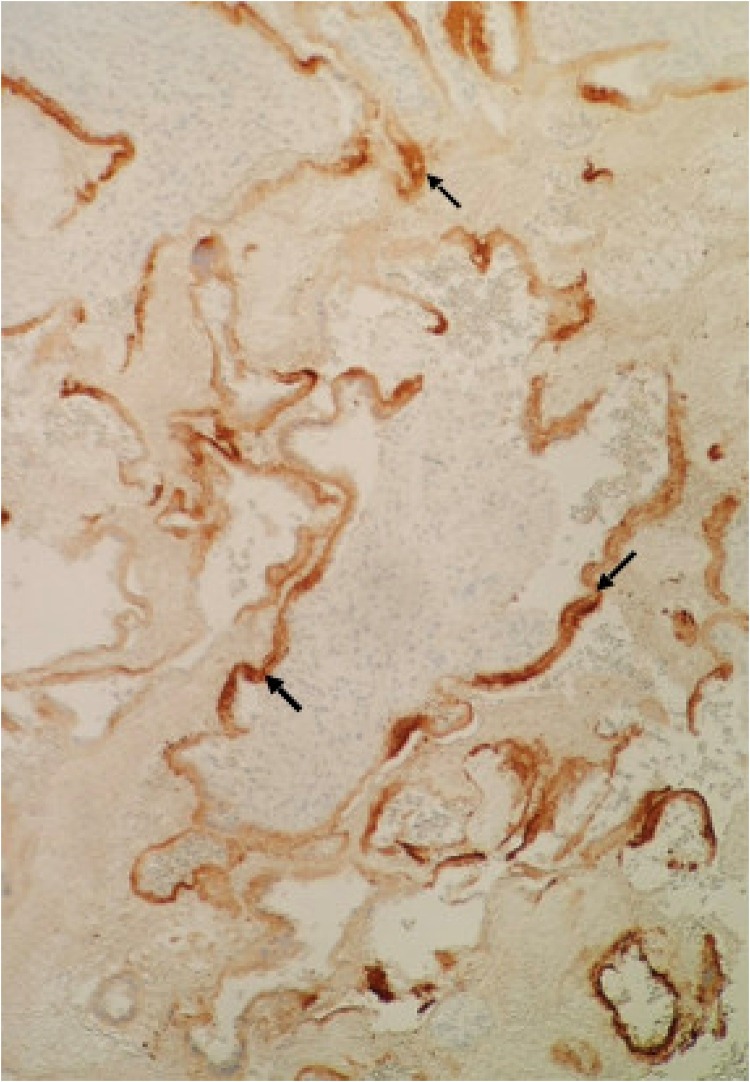
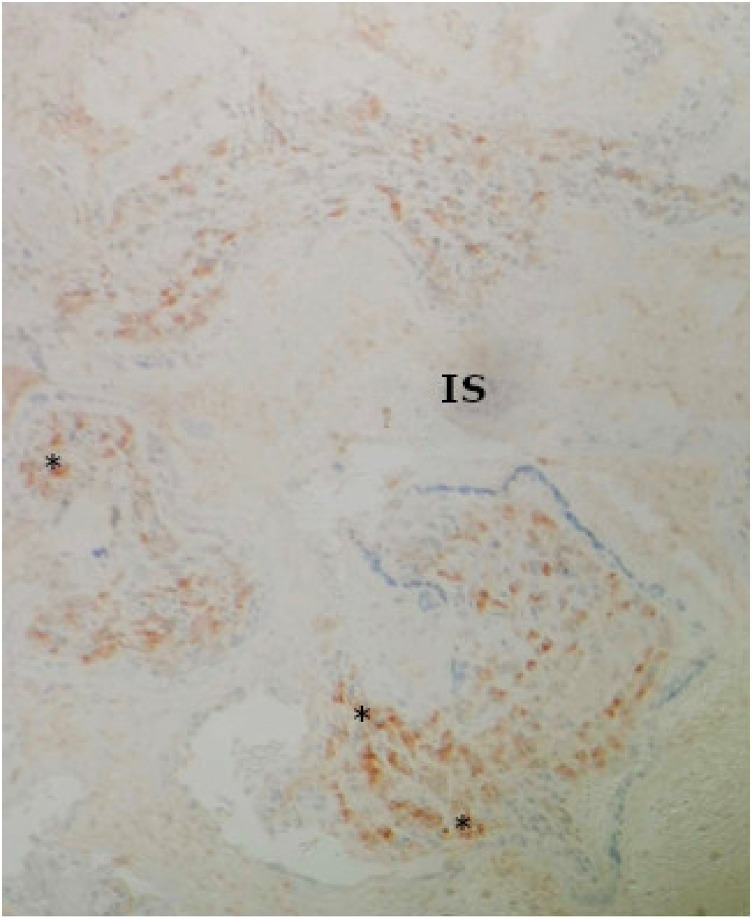
The placental pathology examination found ischemic-hemorrhagic lesions without evidence of viral infection or fetal inflammatory response on 6 different sections with hematoxylin-eosin staining. Specific immunostaining of SARS-CoV-2 nucleoprotein (MyBioSource, San Diego, California) reports a syncitiotrophoblastic staining of a group of villi as previously described [12] (Fig. 1 ). In those specific villi, the number of histiocytes was slightly increased, as shown by CD68 immunostaining. Thus, there was a reactive villitis specifically in infected villi but no intervillitis (Fig. 2 ). The fetal autopsy found a fetus with normal biometry for 16 weeks of gestation and no congenital malformation. Negative RT-PCR for SARS-CoV-2 findings for the fetal liver and lung showed the absence of vertical transmission to the fetus (Table 1).
Fig. 1.
Histopathology of placenta. Syncitiotrophoblastic staining by SARS-CoV-2 nucleoprotein ( ) of a group of villi. (×10).
) of a group of villi. (×10).
Fig. 2.
Histopathology of placenta. Increased number of intravillous histiocytes (*) identified by CD68 immunohistochemical staining, absence of histiocytes in the intervillous space (IS). (×10).
Discussion
We report a case of fetal loss that may be attributed by the infection of the placenta by SARS-CoV-2. There is a physiological possibility that SARS-CoV-2 may cause prematurity or fetal loss [4].
Indeed, several non-genital tract infections, such as pyelonephritis, asymptomatic bacteriuria, pneumonia, and appendicitis are associated with preterm birth [9]. Amniochorial infection has been found to be associated with preterm birth or fetal loss [13].
More specifically pPROM is a disease of the fetal membranes where the inflammation–oxydative stress axis, potentially induced by amniochorial infection, plays an important role in producing the pathways that can result in membrane rupture [14].
In this case, the infection by SARS-CoV-2 of the placenta may have induced a fetal loss through a local inflammation. That may have been favored by the placental expression of the receptor of angiotensin-converting enzyme 2 (ACE2) used as the entry point to host cells by the SARS-CoV-2 [15]. Narang and al report that, because of higher ACE2 receptor expression, pregnant women may be at elevated risk for complications from SARS-CoV-2 infection. [16]
Data regarding preterm birth and SARS-CoV-2 are still limited. A prospective national population based cohort study using the UK Obstetric Surveillance System (UKOSS) including 427 women reported 25 % of preterm birth in women hospitalized for COVID-19 [17]. A case series of pregnant women with COVID-19 in a research network of 33 French maternity units including 620 women reported 48 % of preterm birth in women requiring oxygen [18]. Compared with women without the disease, those with COVID-19 may have an increased risk of preterm birth (OR 3.0 ; 95 % CI 1.2–7.9) [19]. However, whether the prematurity is spontaneous or induced has not been properly investigated.
In the 3rd trimester, cases were described of probable transmission of SARS-CoV-2 from women infected with COVID-19, in particular the case described by Vivanti and al [20] reporting a patient at 35 and 2 weeks of gestation infected by SARS-CoV-2 who presented severe fetal heart abnormalities on the third day of her hospitalization which indicated a cesarean section. Placental and amniotic fluid virological analysis revealed a positive SARS-CoV-2 RT-PCR. The child was immediately placed in isolation presented neurological signs on the third day of life, the etiological analysis of which only found a SARS-CoV-2 infection. This could be an element in favor of a probable placental transmission of SARS-CoV-2.
There is only one other description of fetal loss, very similar to ours, associated with a placental infection by SARS-CoV-2 [10]. This case reports a symptomatic 28-year-old woman, with a positive SARS-CoV 2 PCR who had a fetal loss at 19 weeks of gestation. She had a late miscarriage like our patient. Differences between the two cases are: i) In their case, the patient did not have a history of full-term delivery like our patient; ii) Their health care professionals were tested negative for SARS-CoV-2. We did not perform this test in our hospital because health care providers were asymptomatic and wore a mask with the patient. iii) They found a negative vaginal swab for SARS-CoV-2, but we did not take the test because our patient came to the hospital during labor. Histopathological analyses were similar. In our case, numerous histiocytes were found on CD68 immunology within a few villi related to reactive villitis but no sign of intervillitis unlike the case described by Baud and al [10]. The marked villi were only the COVID positive ones, but not all COVID positive villi were marked probably due to an early stage in the inflammatory response.
The main point of discussion is the causal relation between the placental infection by SARS-CoV-2 and the pregnancy loss. Although the only cause found in this case was a placental SARS-CoV-2 infection, we can not completely rule out other undiagnosed causes of fetal loss.
In conclusion, this case illustrates a potential impact of SARS-CoV-2 among pregnant women. Specific investigations of the consequences of COVID-19 infection on obstetric outcomes are needed.
References
- 1.Galang R.R., Chang K., Strid P., Snead M.C., Woodworth K.R., House L.D., et al. Severe coronavirus infections in pregnancy: a systematic review. Obstet Gynecol. 2020;136(2):262–272. doi: 10.1097/AOG.0000000000004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L., et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capobianco G., Saderi L., Aliberti S., Mondoni M., Piana A., Dessole F., et al. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:543–558. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalagiri R.R., Carder T., Choudhury S., Vora N., Ballard A.R., Govande V., et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33(14):1337–1356. doi: 10.1055/s-0036-1582397. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh H., Way S.S. Immunological basis for recurrent fetal loss and pregnancy complications. Annu Rev Pathol. 2019;14:185–210. doi: 10.1146/annurev-pathmechdis-012418-012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hachem R., Markou G.A., Veluppillai C., Poncelet C. Late miscarriage as a presenting manifestation of COVID-19. Eur J Obstet Gynecol Reprod Biol. 2020;252:614. doi: 10.1016/j.ejogrb.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmo F.R., Alves E.A.R., Moreira R.A.A., Severino V.O., Rocha L.P., Monteiro M., et al. Intrauterine infection, immune system and premature birth. J Matern Fetal Neonatal Med. 2018;31(9):1227–1233. doi: 10.1080/14767058.2017.1311318. [DOI] [PubMed] [Google Scholar]
- 14.Menon R., Richardson L.S. Preterm prelabor rupture of the membranes: a disease of the fetal membranes. Semin Perinatol. 2017;41(7):409–419. doi: 10.1053/j.semperi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narang K., Enninga E.A.L., Gunaratne M., Ibirogba E.R., Trad A.T.A., Elrefaei A., et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin Proc. 2020;95(8):1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayem G., Lecarpentier E., Deruelle P., Bretelle F., Azria E., Blanc J., et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod. 2020;49(7) doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;(370):m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]