Fig. 1. Ubiquitination of IKKβ inhibits the phosphorylation of p105.
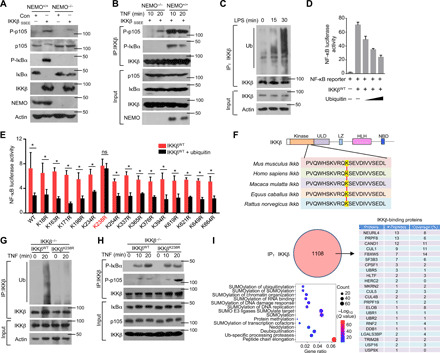
(A) NEMO-deficient MEFs were reconstituted with the indicated constructs. The Western blot analysis results of p105 and IκBα phosphorylation and steady-state expression levels in these cells are shown. (B) By using these reconstituted MEFs, IKK kinase activity on different substrates was determined with an in vitro kinase assay in the presence of GST-IκBα or GST-p105. (C) Mouse BMDMs isolated from WT mice were stimulated with LPS. Whole-cell lysate (WL) was subjected to IP using an anti-IKKβ antibody, which was followed by IB analysis of the ubiquitination (Ub) level. (D) HEK293T cells were transfected with NF-κB luciferase reporters along with IKKβWT and ubiquitin expression plasmids. The readouts were normalized to Renilla luciferase activity and are presented as the fold changes relative to the values in untransfected cells. (E) IKKβWT (WT) and multiple site mutants were transfected into HEK293T cells. After 8 hours of TNF-α treatment, the cells were lysed for luciferase assays. ns, not significant. (F) Sequence alignment of Ub sites on IKKβ orthologs of different species. (G) IKKβWT and IKKβK238R were reconstituted into IKKβ−/− cells. WLs were subjected to IP using anti-IKKβ followed by Ub analysis. (H) IKK kinase activity was determined by kinase assays upon IP with an anti-IKKβ antibody. (I) HEK293T cells were transfected with HA-IKKβ and subjected to IP using anti-HA before mass analysis. The associated proteins of IKKβ are presented as indicated. The bars and error bars show the means ± SEMs. The significance of the differences in (E) was determined by the two-tailed Student’s t test. *P < 0.05.
