Fig. 4. USP16-mediated DUB of IKKβ is required for its binding to p105.
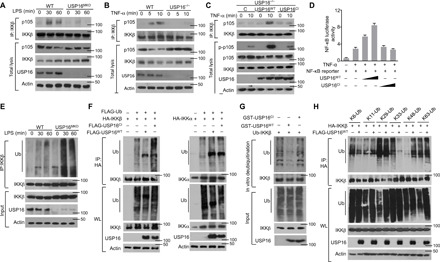
(A and B) The interaction between IKKβ and p105 was assessed in WT and USP16-deficient BMDMs stimulated by LPS (1 μg/ml) (A) or in TNF-α–treated USP16−/− MEFs (B) via IP with an anti-IKKβ antibody. (C) USP16-deficient MEFs were reconstituted with WT or catalytically inactive USP16. IB analysis of the interaction between IKKβ and p105 under TNF-α (50 ng/ml) stimulation was performed as indicated. (D) HEK293T cells were transfected with an NF-κB–luciferase reporter plasmid in the presence (+) or absence (−) of the indicated empty vector or expression plasmids. Luciferase assays were performed, and the results are presented as fold changes based on the empty vector group 36 hours after transfection. (E) IKKβ was isolated by IP (under denaturing conditions) from WLs of WT and USP16-deficient BMDMs and subjected to IB assays using anti-ubiquitin (top). Protein lysates were also subjected to direct IB (bottom). (F) HEK293T cells were transfected with HA-tagged ubiquitin along with the indicated expression plasmids. The ubiquitination levels of IKKβ and IKKα were examined by IB. Cell lysates were also subjected to direct IB (bottom three). (G) In vitro assays were used to evaluate USP16-mediated IKKβ DUB. Recombinant WT or inactive USP16 (USP16mut) was incubated with ubiquitinated IKKβ isolated from transfected HEK293T cells. Ubiquitination was detected by IB. (H) HEK293T cells were transfected with multiple ubiquitin mutants (mutations at K6, K11, K48, K63, K29, and K33) and the indicated expression plasmids. HA-tagged IKKβ was isolated by IP, and the ubiquitination level was then detected by IB. The data are representative of at least three independent experiments.
