Abstract
Historically, the focus of type II diabetes mellitus (T2DM) research has been on host metabolism and hormone action. However, emerging evidence suggests that the gut microbiome, commensal microbes that colonize the gastrointestinal tract, also play a significant role in T2DM pathogenesis. Specifically, gut microbes metabolize what is available to them through the host diet to produce small molecule metabolites that can have endocrine-like effects on human cells. In fact, the meta-organismal crosstalk between gut microbe-generated metabolites and host receptor systems may represent an untapped therapeutic target for those at risk for or suffering from T2DM. Recent evidence suggests that gut microbe-derived metabolites can impact host adiposity, insulin resistance, and hormone secretion to collectively impact T2DM progression. Here we review the current evidence that structurally diverse gut microbe-derived metabolites, including short chain fatty acids, secondary bile acids, aromatic metabolites, trimethylamine-N-oxide, polyamines, and N-acyl amides, that can engage with host receptors in an endocrine-like manner to promote host metabolic disturbance associated with T2DM. Although these microbe-host signaling circuits are not as well understood as host hormonal signaling, they hold untapped potential as new druggable targets to improve T2DM complications. Whether drugs that selectively target meta-organismal endocrinology will be safe and efficacious in treating T2DM is a key new question in the field of endocrinology. Here we discuss the opportunities and challenges in targeting the gut microbial endocrine organ for the treatment of diabetes and potentially many other diseases where diet-microbe-host interactions play a contributory role.
Keywords: diabetes, microbiome, metabolism, nutrition
Over the past 30 years, the number of people with T2DM and prediabetes has increase 2-fold globally, positioning T2DM as a rapidly expanding public health challenge. T2DM is a multifactorial disease that slowly progresses over many years (Fig. 1), and as such therapeutic strategies are often designed in a personalized manner according to what stage of progression the patient presents. Although there are clear host genetic determinants impacting the global T2DM epidemic, genetic variation accounts for only a small proportion of risk of developing T2DM. In addition to genetic predisposition, unhealthy diets and a sedentary lifestyle are key drivers of the early phases of prediabetes, insulin resistance, and low-grade chronic inflammation that drive diabetic complications. Clearly, poor dietary practices play a predominant role in slow progression of prediabetes to frank T2DM. Therefore, understanding how dietary factors drive T2DM pathogenesis will be central in drug discovery moving forward.
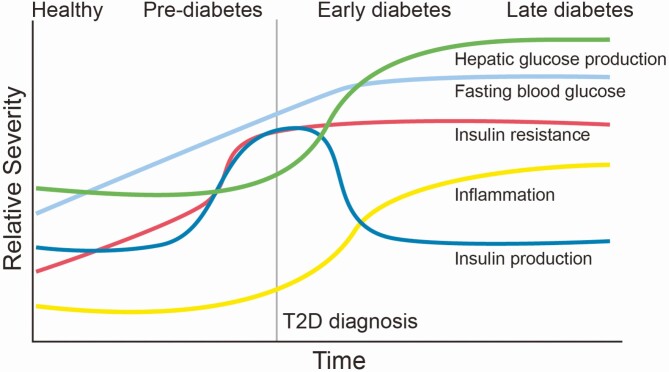
Figure 1.
The chronological progression of T2DM. Over a chronic period of poor diet and sedentary lifestyle, the clinical course of T2DM occurs. In the early phase of transitioning from health to the prediabetic states there is a gradual rise in tissue insulin resistance, small increases in blood glucose, and gradual increases in low-grade systemic inflammation. As insulin resistance persists in early prediabetes pancreatic β cells compensate to overproduce insulin in an attempt to maintain euglycemia. For a period of time insulin overproduction in the pancreas can compensate, but with long-term tissue insulin resistance, inflammation, and lipotoxicity, β cells die, and insulin production drops as a result. In the later stages of disease progression, fasting blood glucose is uncontrollably elevated as a culmination of uncontrolled hepatic glucose production, defective insulin production, and tissue insulin resistance. The resulting hyperglycemia, associated chronic low-grade inflammation, and tissue lipotoxicity contribute to tissue injury and downstream diabetic complications.
Here we discuss how common nutrients in our diets can participate in meta-organismal (microbe to host) endocrine signaling pathways to promote T2DM progression. It is largely assumed that nutrients from our diets are simply absorbed or metabolized by our human cells primarily for energy needs and general cell health. However, there is clear evidence that microbes resident in the human gastrointestinal tract play major roles not only in allowing us to efficiently harvest energy from our food, but also serve as a key endocrine organ producing small molecule metabolites that act in a hormone-like manner to impact T2DM pathogenesis. It is important to understand that gut microbe-derived metabolites are sensed by dedicated receptor systems in the human host, and these host receptors may represent interesting new therapeutic targets (Fig. 2). Gut microbes can also signal to the host to regulate T2DM through direct microbe-host interactions, where constituents of the microbial cell wall are sensed by host cells through pattern recognition receptors (PRR) to promote or protect against inflammatory processes associated with T2DM complications (Fig. 3). Specifically, toll-like receptors (TLRs) 2 (as a heterodimer with either TLRs 1 or 6), 3, and 4 as well as nucleotide-binding oligomerization domain-containing protein 1 (NOD1) have been mechanistically shown to exacerbate inflammation in diet-induced models of inflammation (1-6). Expression of TLRs 7, 8, and 9 have also been associated with diabetes in various contexts of obesity and T2DM (7-9). On the other hand, signaling through TLR5 and NOD1 have shown to be protective against insulin resistance (10-15) (Fig. 3). Collectively, through both nutrient metabolism-dependent (i.e., metabolite-driven) and metabolism-independent (i.e., PRR-driven) mechanisms, the gut microbiome forms a largely overlooked endocrine organ that plays a key role in the progression of T2DM. With our growing understanding of diet-microbe-host interactions, there is rapidly expanding potential that drugs targeting meta-organismal endocrinology may be a provocative new way to advance T2DM drug discovery.
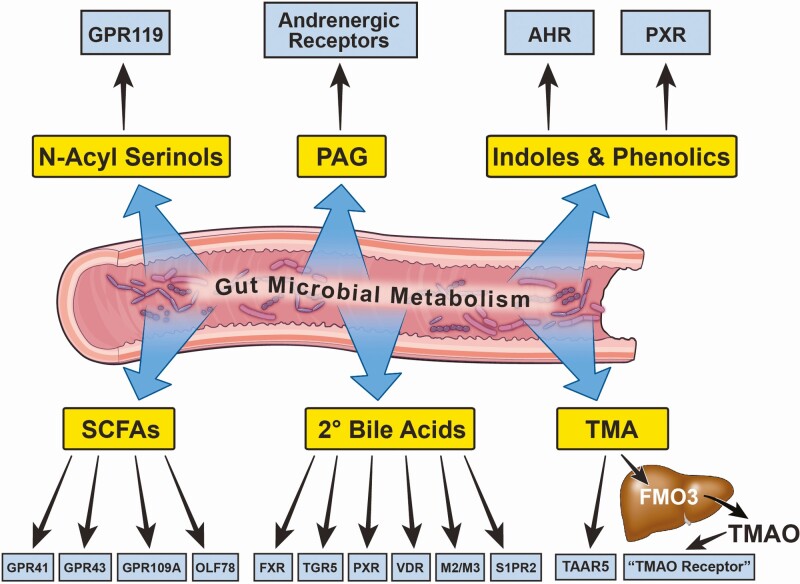
Figure 2.
Gut microbial metabolites as hormone-like signals contributing to T2DM. After a meal provides the necessary substrates, gut microbes produce unique metabolites that are sensed by the host through dedicated receptor systems to elicit a biological response. Given these metabolites are generated in the large bowel and travel via the circulation to engage host receptors, these bacterially derived metabolites fit all of the criteria of a “hormone.” Host receptors have been identified for microbial metabolite-driven pathways that signal to reorganize host metabolism and inflammation to alter T2DM progression. Abbreviations: AHR, aryl hydrocarbon receptor; FMO3, flavin monooxygenase 3; FXR, farnesoid X receptor; GPR41, G protein-coupled receptor 41; GPR43, G protein-coupled receptor 43; GPR109A, G protein-coupled receptor 109A; GRP119, G protein-coupled receptor 119; M2/M3, muscarinic receptors 2/3; TGR5, G protein-coupled bile acid receptor 1; OLF78, olfactory receptor 78; PAG, phenylacetylglutamine/phenylacetylglycine; PXR, pregnane X receptor; SCFA, short chain fatty acids; S1PR2, sphingosine-1-phosphate receptor 2; T2DM, type II diabetes mellitus; TAAR5, trace-amine associated receptor; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; VDR, vitamin D receptor; 2o bile acids, secondary bile acids.
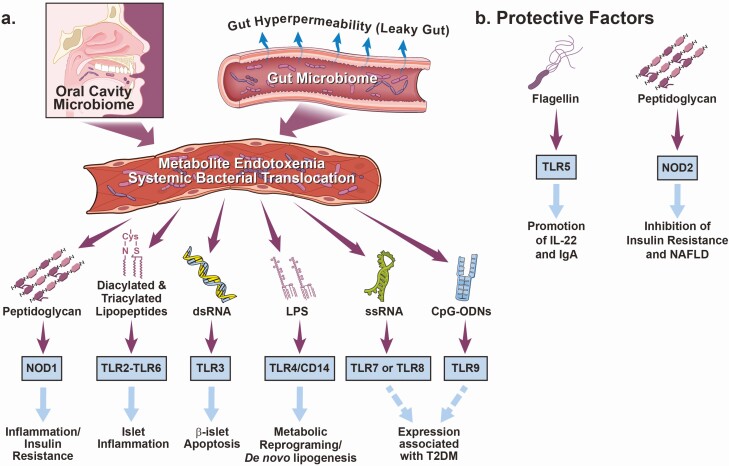
Figure 3.
Direct engagement of pattern recognition receptors by microbial-associated molecular patterns play a role in T2DM. (A) Microbial-associated molecular patterns (MAMPs) can contribute to T2DM via the direct engagement of host pattern recognition receptors (PRRs), promoting chronic low-grade inflammation in insulin producing and insulin sensitive tissues. In the context of T2DM, gut microbial communities are significantly altered and associated gut hyperpermeability can elicit local direct MAMP-PRR signaling within key tissue microenvironments (gut, liver, pancreas, adipose, kidney, etc.). This MAMP-PRR signaling can promote tissue inflammation, which can contribute to the complications of T2DM. (B) Conversely, some MAMPS and PRRs are implicated in protecting against T2DM. Abbreviations: CD14, cluster of differentiation 14; CpG ODNs, CpG oligodeoxynucleotides; LPS, lipopolysaccharide; MAMPs, microbial-associated molecular patterns; MI, myocardial infarction; NOD1, nucleotide oligomerization domain-containing 1; NOD2, nucleotide oligomerization domain-containing 2; T2DM, type II diabetes mellitus; TLR, toll-like receptor.
Type II Diabetes Mellitus Etiology, Pathogenesis, and the Role of Host Hormonal Dysfunction
The incidence of T2DM continues to increase in the United States and globally as the abundance of food in the developed world leads to chronic overnutrition and sedentary lifestyles becomes commonplace (16,17). Fundamentally, T2DM is a disease of improper fasting/refeeding responses controlled by a complex host endocrine system, resulting in improper control of glucose and lipid metabolism that then contributes to many T2DM-associated complications. Clinically, diabetes can be defined by different measurements of blood glucose including hemoglobin A1c (5.7%-6.4% considered prediabetic and >6.5% is diabetic) and fasting plasma glucose (100-125mg/dL considered prediabetic and >125 mg/dL is diabetic) (18). Despite the primary role of environmental factors such as smoking, poor diet, and sedentary lifestyle in T2DM development and progression, the basis for genetic susceptibility has also been investigated (19-21). As might be expected, many genes regulating host metabolism and hormonal regulation of glucose and lipid metabolism have been associated with T2DM (20). However, genetic variation accounts for only a small proportion of risk of developing T2DM, and environmental factors play a predominant role in driving the progression of this slowly developing disease (Fig. 1). In addition to genetic predisposition, the pathogenesis of T2DM begins with chronic nutrient excess in a healthy individual at baseline, leading to increased weight gain (i.e., adiposity) as the body is unable to utilize all the nutrients it is receiving. With the expansion of adipose tissue, insulin resistance develops resulting in reduced nutrient uptake, moderate hyperglycemia, and dyslipidemia (22). Additionally, as the lipid storage capacity of adipose tissue is finite, in obese individuals other nonadipose tissues such at the liver and skeletal muscle can begin to take on ectopic lipids which can promote lipotoxicity and associated insulin resistance. During the early phase of T2DM progression, pancreatic β cells can increase the production of insulin to counteract tissue insulin resistance. However, over time pancreatic β cells exhibit reduced capacity for glucose-stimulated insulin secretion and, in some cases, die, leading to loss of insulin production and eventually dependence on exogenous insulin (Fig. 1).
In addition to damage in the liver, adipose tissue, skeletal muscle, and pancreas, pathology develops in the vasculature and kidneys. These pathological aberrations contribute to complications associated with T2DM such as nonalcoholic fatty liver disease (NAFLD), cardiovascular disease, chronic kidney disease (CKD), diabetic retinopathy, etc. NAFLD exists on a spectrum between simple hepatic steatosis to cirrhosis and end organ failure. As such, ectopic fat accumulation in the liver because of adipose tissue insulin resistance and then immune activation from extracellular nutrients contribute to NAFLD development and progression. Diabetic dysregulation of circulating lipids (i.e., dyslipidemia) may lead to lipid accumulation in artery walls (i.e., atherosclerosis) and dysregulated blood pressure putting patients at risk for major adverse cardiovascular events such as heart attack and stroke. Collectively, the gradual progression from prediabetes to frank T2DM promotes downstream tissue injury over a period of decades (Fig. 1), and now T2DM is thoughtfully managed with a combination of drugs and lifestyle modifications.
It is important to note that there have been game-changing advances (23, 24) in the treatment and management of all phases of T2DM, particularly with the advent of recombinant insulin and effective insulin sensitizing drugs. Therapeutic options for T2DM vary from lifestyle to pharmacological to surgical interventions. Specifically, lifestyle modification includes improving diet to restrict excess nutrient intake and increasing physical activity, while pharmacological treatment options are represented by recombinant insulin peroxisome proliferator-activated receptor gamma agonists (“glitazones”), sulfonylureas, metformin, sodium-glucose cotransporter-2 inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists. In the common case where T2DM develops in obese subjects, surgical options such as bariatric surgery can show dramatic improvements in diabetic complications. Historically, host derived hormones and hormonal control of host metabolism have been the focus of T2DM drug discovery. More recently, the investigation of the gut microbiota in host health and disease has exploded, ushering in a new wealth of information and potential for novel therapies that target meta-organismal endocrinology. It has become clear that gut microbes act as their own endocrine organ, communicating among each other and with their host in response to dietary cues. As such, there have been numerous studies implicating various microbial metabolites in T2DM pathogenesis. Despite many emerging studies, there remains a need to identify novel microbe-host interactions that correlate with T2DM outcomes, determine the mechanisms by which these novel and previously identified metabolites exert an effect on disease, and design novel therapies aimed at targeting the microbiota to modulate the production of these metabolites and downstream mechanisms to limit disease progression. Here we discuss meta-organismal endocrine pathways implicated in T2DM and the potential molecular mechanisms and therapeutic potential.
Gut Microbe-Derived Short Chain Fatty Acids in T2DM
The short chain fatty acids (SCFAs), acetate, propionate, and butyrate are metabolic products of soluble dietary fiber and amino acids, were the first class of microbiota-derived metabolites to be thoroughly investigated as having implications in T2DM. Total SCFA concentrations have been measured micromolar concentrations in both portal and peripheral blood of humans (25). Four G-protein coupled receptors (GPCRs) for SCFAs have been identified: GPR41 (free fatty acid receptor 3), GPR43 (free fatty acid receptor 2), GPR109a, and olfactory receptor 78 (26-39) (Fig. 2). Of the 4 receptors, the 2 most directly implicated in T2DM are GPR41 and GPR43. GPR41 recognizes all 3 SCFAs but is activated 10-fold less by acetate and is reported to be expressed in adipose tissue, the gut by intestinal L-cells, the nervous system, and the kidney (26,34,36). Although GPR41 was reported to be expressed in mouse adipose tissue and induce leptin secretion (27), other studies have failed to find expression in this tissue (30,40). GPR41 knockout resulted in increased gut motility via reduced peptide YY (PYY) secretion, which resulted in decreased intestinal absorption of nutrients, implicating a role in food consumption (28). Consistent with this role in regulating food consumption, in the sympathetic nervous system, GPR41 activation was found to stimulate energy expenditure, thereby having a protective effect against T2DM onset (30). In the kidney, GPR41 seems to play a role in decreasing blood pressure, which could play a protective role againstT2DM-mediated kidney damage in later disease stages (34). Overall, these effects suggest activation of GPR41 has a beneficial effect on T2DM in murine models. However, there is limited information available regarding GPR41 in human tissues.
GPR43 has been shown to be activated equally by all SCFAs and is expressed in adipose tissues, the liver, the intestines, skeletal muscle, and the kidney (26,31,34,35). In the context of high fat diet, GPR43 has been found to be upregulated in adipose tissue, skeletal muscle, and the liver (31,40). Murine transgenic models of GPR43 have shown improved metabolic parameters such as reduced adiposity, increased lean mass, improved glucose homeostasis, and higher GLP-1 secretion (29,32,33). On the other hand, GPR43 activation with acetate and propionate was shown to promote adipocyte differentiation and neutral lipid accumulation in vitro (40). Additional investigation is needed to understand the circumstances by which GPR43 signaling has varying results in animal and cell culture models as well as the receptor’s role in the kidney. Like GPR41, the understanding of GPR43’s role in T2DM is difficult to interpret due to limited information about the human receptor as well as some confounding findings when comparing mouse to human GPR43 (41).
Another SCFA receptor known as GPR109a is expressed on innate immune cells, adipocytes, and intestinal epithelial cells to respond to butyrate and niacin (vitamin B3), reducing inflammatory responses (37,39). Specifically, GPR109a knockout mice show changes in colonic T-cell and innate immune cell populations as well as reduced epithelial function (37,39). Despite its established role in intestinal inflammation, GPR109a function has not been investigated in-depth thus far in T2DM models or adipose tissue but may be important in limiting the chronic low-grade inflammation associated with T2DM (Fig. 1). The last GPCR for SCFAs, Olf78, has been observed in the olfactory bulb, enteroendocrine cells, the kidney, blood vessels, skeletal muscle, and heart (34,42). Olf78 seems responsive to acetate and propionate, but not butyrate, and induces an increase in blood pressure via renin secretion (34). As such, Olf78 has been proposed to have the opposite effects on blood pressure versus agonism of GPR41, suggesting that a bimodal response is possible depending on the concentration of SCFAs and the activation of these 2 receptors (34). If true, such a mechanism may account for some inconsistencies reported in the effects of SCFAs. For example, Olf78 activation may antagonize the action of GPR41 activation in the intestine, thereby decreasing PYY secretion overall. Further studies focused on the redundant and antagonistic actions of different SCFA receptors and using human receptors are needed to tease out their specific effects and importance in T2DM pathology.
In addition to studies focused specifically on the GPCRs sensing these SCFAs, direct effects of propionate, acetate, and butyrate have been investigated. Overall these studies show that all 3 SCFAs have effects on lipid metabolism showing reduced hepatic and visceral adiposity (38,43-47). In addition to these effects, propionate was shown to stimulate PYY and GLP-1 release thereby reducing energy intake, acetate induced browning in adipose tissue, and butyrate was shown to rescue decreased GLP-1 receptor expression in the liver and decrease inflammation (38,45-48). Consistent with the previously discussed studies on SCFA receptors, treatment with SCFAs in both rodent models and humans improves T2DM phenotypes (38,45-48).
In addition to the previously discussed SCFAs, branched chain fatty acids (BCFAs), which are produced from branched chain amino acids (valine, leucine, and isoleucine) in Bacteroides, Eubacterium, and Clostridium species have also gained attention (49-51). These studies have suggested that BCFAs suppress immunoglobin A production and modify insulin resistance and signaling both positively and negatively (50-52). More investigation will be needed better understand the conflicting reports of BCFAs’ effects on metabolism. Overall, SCFAs seem to improve metabolic parameters, but given their varied receptors and effects in multiple tissues these observations may not comprehensively describe the role of SCFAs in T2DM development and pathogenesis. Additionally, the production of BCFAs by the same bacterial genera as SCFAs may complicate in vivo findings.
Microbial Bile Acid Metabolism in T2DM
Primary bile acids (1BAs), cholic acid and chenodeoxycholic acid, are produced by the liver, conjugated with either glycine or taurine, and secreted from the gallbladder into the intestinal tract where most are reabsorbed and circulate at nanomolar to low micromolar concentrations (53). A small portion reaches the lower intestinal tract where microbes cleave the glycine or taurine and either reduce or epimerize the 7-hydroxy group to make the secondary bile acids (2BAs): deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA), which can then be absorbed into portal circulation, reaching nanomolar concentrations (53,54) (Fig. 4). Both 1BAs and 2BAs may are poised to play a central role in T2DM pathogenesis given their functions in intestinal fat and vitamin absorption and glucose homeostasis (55). These bile acids bind to and activate nuclear hormone receptors, such as the farnesoid X receptor (FXR), pregnane X receptor (PXR), and vitamin D receptor (VDR), as well as G-protein-coupled bile acid receptor-1 (TGR5) (Fig. 4). FXR is a nuclear hormone receptor that is expressed in the liver, intestines, kidney, and several other tissues where it regulates bile acid homeostasis in addition to lipid and glucose metabolism in response to both 1BAs and 2BAs (56). For 2BAs, FXR responds to DCA and LCA to negatively regulate bile acid production through the small heterodimer partner and fibroblast growth factor-15/19 (FGF15/FGF19) that inhibit expression of bile acid synthetic enzymes cytochrome P450 family 7 subfamily A member 1and cytochrome P450 family 8 subfamily B member 1 (57). In diabetic mice, the administration of a synthetic FXR agonist markedly reduced plasma glucose, triglycerides, free fatty acids, and cholesterol as well as hepatic steatosis (56). The effect on glucose homeostasis was attributed to improved insulin sensitivity, while decreased triglycerides resulted from reduced sterol regulatory-binding protein 1c, and increased reverse cholesterol transport was achieved through the enhanced expression of lecithin:cholesterol acyltransferase and scavenger receptor class B type 1 (56). Conversely, intestine-specific knockout of FXR in mice was shown to reduce insulin resistance and hepatic triglyceride accumulation (58). Most recently, it was demonstrated that an intestinally targeted pharmacological agonist of FXR promoted profound improvements in insulin resistance and enhanced energy expenditure in mice (59). Although there are some evidence supporting FXR agonists as antidiabetic agents, further study of FXR signaling in T2DM pathogenesis is warranted due to discrepant results across divergent preclinical animal model studies.
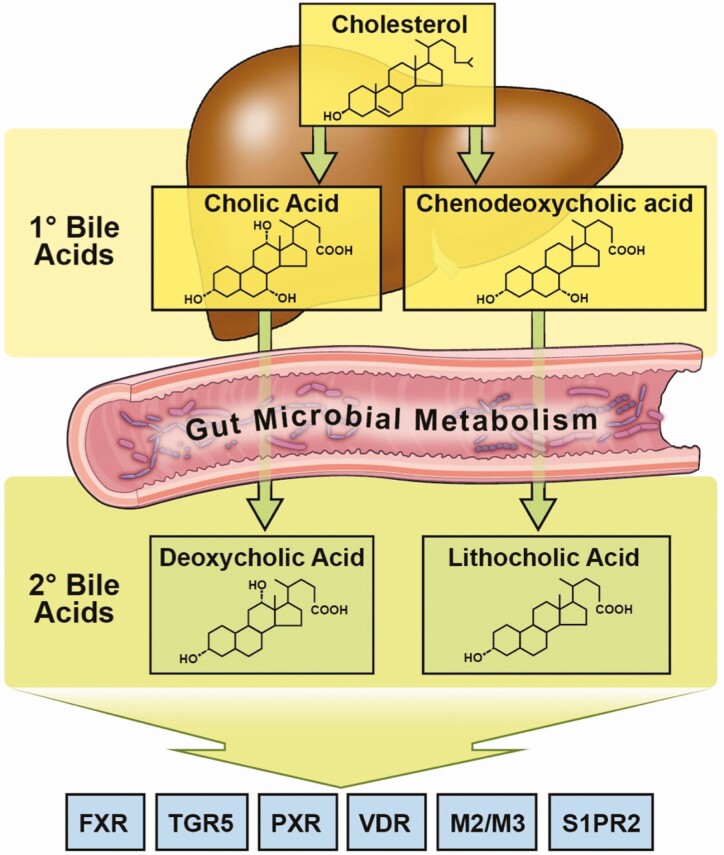
Figure 4.
Meta-organismal bile acid metabolism as a contributor to T2DM progression. Primary bile acids are synthesized in the host liver from cholesterol. De novo synthesized primary bile acids such as cholic acid, chenodeoxycholic acid (CDCA) and muricholic acid (MCA; only produced in rodents) are then conjugated with either glycine (humans) or taurine (humans and mice). Following conjugation, resulting bile salts are secreted into bile along with cholesterol and phospholipids to form mixed micelles, which are transiently stored in the gall bladder. When a meal is ingested, the gall bladder contracts to release mixed micelles into the proximal intestine where they function as essential emulsifiers to enable proper absorption of hydrophobic molecules such as fatty acids and fat-soluble vitamins. Importantly, bile salts are left behind in the intestinal lumen where they ultimately traverse to the colon, where they are subsequent to microorganism-driven metabolism of primary bile salts into secondary bile acids (deoxycholic acid and lithocholic acid), which can have an impact on host physiology and disease susceptibility. Importantly, after aiding in intestinal lipid absorption both primary bile salts and secondary bacterial metabolites are almost quantitatively re-absorbed (>95% recovered) in the ileum via dedicated host transporters in ileal enterocytes. This re-absorptive process provides newly diversified bile acid species, which can signal to the host through dedicated receptor systems, including farnesoid X receptor (FXR), protein-coupled bile acid receptor 1 (TGR5), pregnane X receptor (PXR), vitamin D receptor (VDR), muscarinic receptors 2 and 3 (M2/M3), and sphingosine-1-phosphate receptor 2 (S1PR2). By both regulating intestinal lipid absorption and host receptor activation both primary and secondary bile acids can have profound effects on T2DM progression.
PXR was shown to upregulate a detoxification network in response to LCA, which involves downregulation of cytochrome P450 family 7 subfamily A member 1 to decrease the production of bile acids as well as upregulation of OATP2 and CYP3A to increase the clearance of bile acids, thereby promoting lipid and fat-soluble vitamin uptake (60). VDR was also shown to be activated by LCA, albeit at a concentration 1000-fold greater than active vitamin D (55). Interestingly, VDR activation by LCA was found to affect T cell activation (61). Despite these observations, it is unclear whether bile acid-dependent activation of these 2 nuclear hormone receptors play a definitive or direct role in T2DM or its comorbidities.
TGR5, unlike the nuclear receptors discussed above, is a GPCR, expressed by digestive, immune, and adipose tissues where it is preferentially activated by LCA and DCA (62-64). In the gut, TGR5 activation leads to secretion of GLP-1 and PYY, thereby having beneficial effects on insulin secretion and satiety (64-66). TGR5 knockout in macrophages and myeloid-lineage cells was found to exacerbate adipose tissue inflammation and insulin resistance, suggesting a protective role in T2DM pathogenesis (67). Furthermore, bile acid-driven activation of TGR5 in adipose tissue can promote thyroid hormone action thereby increasing energy expenditure (68).Given the varying physiological effects of 2BAs in T2DM models, their diversity of receptors and affected tissues warrant further study to determine whether BA metabolism by the microbiota may be targeted therapeutically. Although there has been a vast array of studies measuring microbe-associated bile acid conversion in human disease (69), additional work is needed to understand the specific role that gut microbes play in host bile acid receptor signaling in the early versus late stages of T2DM progression.
Aromatic Amino Acid and Other Aromatic Metabolites Originating From the Gut Microbial Endocrine Organ
Bacteria are known to catabolize plant polyphenols and aromatic amino acids such as tryptophan, phenylalanine, and histidine to produce various aromatic metabolites. Some polyphenol metabolites including phenylpyruvic acid, apigenin, biochanin A, and 4-hydroxyphenylpyruvic acid as well as tryptophan metabolites such as indole, indole 3-propionic acid (IPA), indole 3-acetic acid, and indole 3-carboxaldehyde, have been identified to exert effects through various nuclear hormone receptors: the aryl hydrocarbon receptor (AHR) and PXR (70-75). Polyphenol metabolites and indole have been consistently identified in plasma at nanomolar concentrations; however, indole metabolites such as IPA have been measured at low micromolar concentrations (76,77).The production of microbial AHR agonists was shown to be decreased in the feces of obese vs lean and T2DM vs non-T2DM patients (78). These results were then recapitulated in genetically obese mice as well as high-fat diet (HFD) fed mice and rats. Administration of an exogenous AHR agonist improved glucose and insulin tolerance in HFD mice, which was shown to be through reduced inflammation and increased GLP-1 secretion (78). As with 2BAs, PXR activation by polyphenols and indoles upregulates CYP3A, which affects xenobiotic metabolism and thus may influence on T2DM patients receiving pharmacological therapy.
In terms of associations between microbe-derived aromatics on T2DM, the Finnish Diabetes Prevention Study, identified higher IPA as being associated with decreased likelihood of developing T2DM and better insulin secretion. Promisingly, this association between IPA and development of T2DM was validated in 2 separate Scandinavian populations (79,80). Among obese individuals with at least 1 comorbidity the production of indole, IPA, indole 3-acetic acid, and indole 3-carboxaldehyde is decreased versus lean individuals, suggesting that a change in meta-organismal tryptophan metabolism may occur prior to T2DM onset and thus may contribute to its development (81). Alternatively, it was shown that IPA level are restored following gastric bypass surgery in HFD fed mice, resulting in improved intestinal barrier function (82). Given these observations, microbial tryptophan production decreases throughout the course of T2DM pathogenesis, but direct study of the role of individual metabolites in T2DM is severely lacking, thereby precluding any inferences about their viability as therapeutic targets.
The association of polyphenol metabolites to T2DM risk and outcomes tend to be positive. However, understanding the exact role of dietary polyphenols and their microbe-derived metabolites in vivo is impeded by their complex meta-organismal metabolism. Specifically, polyphenols are variably glycosylated and degraded depending on their dietary source, host metabolism, and microbial metabolism, affecting their bioavailability and bioactivity (83,84). Although the aforementioned nuclear hormone receptors are known to be affected by polyphenol metabolites, it is likely that other sensing mechanisms exist given the chemical diversity of polyphenol metabolites. Before designing therapeutics aimed at modulating polyphenol metabolites, it will be essential to comprehensively understand the mechanisms by which different metabolites act to then target the production of specific metabolites.
Recently, the phenylalanine-derived metabolite phenylacetic acid was found to be conjugated by the human liver to glutamine, making phenylacetylglutamine (PAGln), which circulates in plasma at micromolar concentrations and correlates with overall mortality and diabetes-associated cardiovascular disease in CKD patients (85). In alignment with causality, subsequent reports showed that PAGln acts through three different adrenergic receptors to promote thrombotic responses (86). Overall, these studies show that upon declining kidney function in CKD, the microbiome structure shifts such that there is an increase in phenylacetic acid producing bacteria (containing porA and fldH genes), which causes increased PAGln production, culminating in increased cardiovascular risk due to inappropriate agonism of adrenergic receptors. In advanced T2DM, declining kidney function may lead to increased PAGln, and this diet-microbe-host signaling axis can contribute to diabetes-associated cardiovascular disease (86).
Also, the microbial product of histidine metabolism, imidazole propionate (ImP), was recently shown to be directly involved in insulin resistance (87). ImP is significantly increased in both portal and peripheral blood of T2DM patients to an average of 10 to 15 micromolar vs obese, body mass index–matched nondiabetics with only 5 micromolar (87). Follow-up in murine models revealed that ImP was significantly increased in conventional vs germ-free mice, indicating the microbial origin of the metabolite. Mechanistically, these mouse studies showed that exogenous ImP showed worsened glucose tolerance, increased gluconeogenic enzyme expression, increased S6 kinase phosphorylation, and impaired insulin signaling in multiple tissues on a normal chow diet. In vitro ImP also impaired insulin-stimulated phosphorylation of protein kinase B (Akt) (87). Taken together, these results suggested that ImP activates mTORC1, which was then confirmed in both mouse and human tissues. Future studies may seek to validate these findings and identify therapeutic interventions aimed at limiting ImP production.
The Gut Microbial Trimethylamine-N-oxide Pathway in T2DM
Common commensal bacteria produce the volatile trimethylamine (TMA) by cleaving it from choline, carnitine, betaine, and phosphatidylcholine (88-90). TMA, which smells like rotting fish, is known to activate a host GPCR called trace amine-associated receptor 5 in the olfactory epithelium. However, the role of trace amine-associated receptor 5activation in processes other than olfaction have remained elusive (91). After reaching portal circulation and trafficking to the liver TMA is oxidized to trimethylamine-N-oxide (TMAO) by flavin-containing monooxygenases (FMOs), most predominantly FMO3 where it then reaches peripheral blood at a micromolar concentrations in humans and mice (88,89,92-95). Although most commonly recognized as a contributing factor in atherosclerosis and cardiovascular disease, the TMA-FMO3-TMAO pathway is also strongly associated with T2DM (94,95). An elegant study by Biddinger and colleagues demonstrated that mice with selective hepatic insulin resistance have elevated levels of circulating TMAO (94). This study demonstrated that male mice lacking the insulin receptor in the liver (LIRKO mice) have a profound up-regulation of the TMAO-producing enzyme FMO3 in the liver (94). Furthermore, antisense oligonucleotide–mediated inhibition of FMO3 in LIRKO mice lacking the insulin receptor in the liver was shown to inhibit TMAO production and to protect against the hyperlipidemic and proatherogenic phenotype of these mice (94). In agreement, an independent study showed that dietary supplementation with TMAO can exacerbate glucose intolerance in high-fat–fed mice (96). In addition, recent studies suggest TMAO levels may similarly be associated with the presence and severity of non-alcoholic fatty liver disease (97-99), which can be a contributing factor in insulin resistance. We have also shown that either inhibition or genetic deletion can protect mice from HFD-induced obesity, which is associated with enhanced beiging of white adipose tissue (95). Consistent with the original observation that it correlates with the T2DM comorbidity cardiovascular disease, TMAO was found to contribute to thrombotic events by causing platelet hyperactivity (94,100-102). An additional tool that has been used for studying this meta-organismal pathway is nonlethal inhibitors of TMA lyase enzymes, which have been associated to thrombosis and cholesterol metabolism (103,104). The selective small targeting the reduction of TMA and TMAO may hold promise in treating T2DM and other diabetes-related complications.
Recently, it was shown that at high concentrations TMAO directly binds to and activates PERK, a key effector of the unfolded protein response in the liver, promoting hyperglycemia and metabolic dysfunction (105). Despite the data from these aforementioned studies, the notion of TMAO playing a role as a pathogenic metabolite has been opposed. One editorial proposed some concerns regarding the study looking at platelet reactivity, stating that dietary intake of TMAO and other factors, such as aspirin usage, should have been considered more carefully (106). Another study, using a bidirectional Mendelian randomization analysis, determined that the causal direction between T2DM and the metabolites betaine and choline was negatively causal (107). Conversely, TMAO and carnitine were not associated causally. Instead, T2DM was found to be causally associated to higher plasma TMAO, suggesting that the disease causes the observed changes to the metabolite rather than the metabolite playing a pathogenic role in disease (107). Taken together, it is evident that the bacterial production of TMA and subsequent conversion to TMAO by host FMO3 is related to T2DM pathogenesis and comorbidities. Given the fact that new drugs specifically targeting gut microbial production of TMA are now available (104), future studies should focus on whether these TMA lyase inhibitors can improve outcomes in preclinical models of obesity, T2DM, and related cardiometabolic diseases.
Meta-organismal Polyamine Metabolism in T2DM
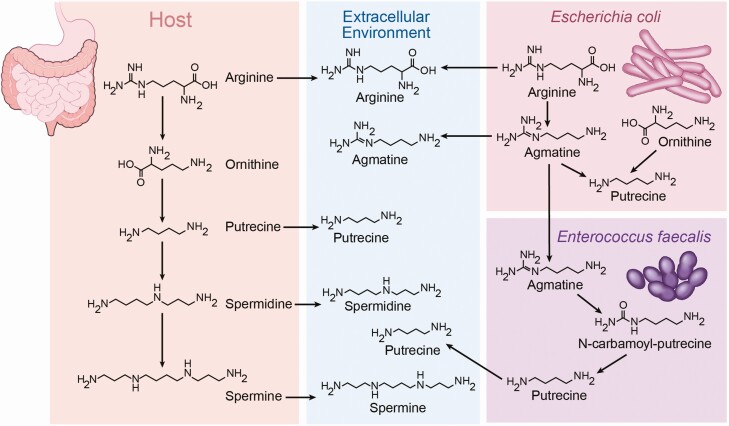
Many catabolite products of lysine, arginine, and ornithine have been identified and collectively referred to as polyamines, which include cadaverine, putrescine, spermine, spermidine, and agmatine (Fig. 5). These metabolites range from nanogram to microgram per milliliter concentrations in human serum (108). It is also important to note that these metabolites can be products of both host and microbe metabolism (108-111). Thus far, not much is known about the role of polyamines in T2DM, although one study found positive correlations between serum putrescine and glycosylated hemoglobin A1c as well as between spermine and fasting insulin (108). Using a functional screening technique, cadaverine, putrescine, and agmatine were identified as activators of the HRH4 histamine receptor from most to least potent (109). A developmental study using a zebrafish model determined that host polyamine synthesis and specifically spermidine was required for proper β-cell differentiation (110). A recent study found that agmatine production by the microbiota in response to metformin is responsible for an increase in fatty acid oxidation in C. elegans, suggesting that a drug-microbe interaction could explain the efficacy of metformin in T2DM patients (111). Lastly, polyamines have been implicated in renal hypertrophy in T2DM and thus may contribute to kidney dysfunction in advanced disease (112). Further research on polyamines in T2DM are warranted, particularly focused on what host sensing mechanisms other than HRH4 are involved and whether altered host and/or microbial polyamine production play functional roles in disease progression.
Figure 5.
The production of polyamines occurs through host and microbial co-metabolism. Gut microbe-derived metabolites of lysine, arginine, and ornithine are collectively referred to as polyamines, which include cadaverine, putrescine, spermine, spermidine, and agmatine, among others. Polyamines can be found in appreciable quantities in the diet, and also polyamine metabolites exhibit complex meta-organismal regulation. As such, precursor metabolites produced by the host may be further metabolized by bacteria to different metabolites. Additionally, microbes may work together to further metabolize the products from neighboring bacteria. Collectively, dietary, microbe-derived, and host-derived polyamine metabolites can contribute to T2DM progression.
Gut Microbial N-acyl Amides as Insulin Sensitizing Hormones
In a recent publication, Cohen and colleagues used functional bacterial metagenomics to discover commensal bacteria that produce N-acyl amides with 6 families of N-acetyl synthase (NAS) genes. These NAS genes acylate amino acids such as glycine, alanine, lysine, and glutamine as well as serinol (113). Many of these N-acyl amides mimic endogenous signaling molecules that bind to host GPCRs (113). Among these GPCRs are G2A, S1PR4, and PTGER4, which are involved in various immune functions as well as GPR119, which has been extensively implicated in T2DM (113-115). Upon cloning type VI NAS into E.coli and monocolonizing mice, it was discovered that the NAS product, N-oleyl serinol, improves glucose tolerance via increased plasma insulin and GLP-1 (113). Work from the same group previously identified commendamide, an N-acylated glycine, as a G2A agonist that activates NF-κB signaling (116). However, whether commendamide signals via G2A to impact T2DM has never been examined (116). Further investigation with native microbes, nonreporter based cell culture, and more in vivo systems are needed to better establish whether these products play a role in T2DM and may be targeted therapeutically.
Conclusions and Future Directions
Significant advances have been made over the past decade to begin elucidating the molecular mechanisms by which these metabolites exert their effects diverging from providing correlation and surrogate readout data that only suggest causality on disease phenotypes. Emerging technology in both preclinical animal models and microbiology will allow for increased proficiency in dissecting the roles of individual metabolites, receptors, and microbes in disease pathology. Given the previous discussion, a trend has emerged that GPCRs tend to be targets of many microbial metabolites; therefore, continued effort should focus on attempting to identify receptors using microbial metabolite libraries and bacterial supernatants (109). Additionally, the implementation of expanding multi-omics platforms and machine learning techniques will aid in the discovery of complex interactions between multiple metabolites. For example, it has recently been shown that the TMA/TMAO pathway likely interacts with the mechanisms surrounding BA metabolism and FXR signaling (103,117). With an enhanced understanding of the gut microbial endocrine organ, therapeutic discovery targeting these interactions may proceed, leading to novel treatment options for T2DM and other diseases.
There is now sufficiently strong evidence that gut microbe-associated pathways play a central role in diet-driven progression of T2DM. Although often overlooked in the field of endocrinology, our gut microbiome functions as a chemically diverse endocrine organ, produces a large number of metabolism-dependent (Fig. 2) and metabolism-independent (Fig. 3) endocrine signals that play regulatory roles in T2DM progression. In simple terms, gut microbiota can serve as a “metabolic filter,” significantly influencing how dietary inputs are assimilated into hormone-like compounds that impact disease pathogenesis. Gut microbiota-derived metabolites discussed here (SCFAs, bile acids, aromatics, polyamines, and N-acyl amides) are clearly altered in T2DM subjects, but whether these metabolites are simply biomarkers of disease or, alternatively, causally linked to disease progression is still an intense matter of debate. To this point the microbiome field has been dominated by correlative metagenomic approaches, simply cataloging microbial communities and predicting metabolic function in disease cohorts is insufficient as we move forward. Instead, functional studies coupling microbial metabolite quantification (metabolomics, proteomics, etc.), dietary manipulation, and host receptor discovery in relevant preclinical animal model systems are essential. Although drug discovery has historically targeted host enzymes and receptors, a fertile period in pharmacology lies ahead where instead we target microbial enzymes (104) or the receptors (GPCRs or nuclear hormone receptors) in the human host that sense gut microbe-derived signals. A central challenge remaining will be to establish mechanistic causal links between bacterial metabolite production and T2DM pathogenesis and to rationally design therapeutic strategies to impact meta-organismal endocrinology.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (NIH): R01 DK120679, P01 HL147823, P50 AA024333, and U01 AA026938. Illustrations were created by David Schumick (BS, CMI) at the Cleveland Clinic Center for Medical Art & Photography.
Glossary
Abbreviations
- 1BAs
Primary bile acids
- 2BAs
secondary bile acids
- AHR
aryl hydrocarbon receptor
- BCFAs
branched chain fatty acids
- CKD
chronic kidney disease
- DCA
deoxycholic acid
- FMO3
flavin monooxygenase 3
- FXR
farnesoid X receptor
- GPCRs
G-protein coupled receptors
- HFD
high fat diet
- ImP
imidazole propionate
- IndS
indoxyl sulfate
- IPA
indole 3-propionic acid
- LCA
lithocholic acid
- NAFLD
non-alcoholic fatty liver disease
- NAS
N-acetyl synthase
- PAGln
phenylacetylglutamine
- PXR
pregnane X receptor
- SCFAs
short chain fatty acids
- T2DM
type II diabetes mellitus
- TMA
trimethylamine
- TMAO
trimethylamine-N-oxide
- UDCA
ursodeoxycholic acid
- VDR
vitamin D receptor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Lancaster GI, Langley KG, Berglund NA, et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 2018;27:1096-1110.e1095. [DOI] [PubMed] [Google Scholar]
- 2. Todoric J, Di Caro G, Reibe S, et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2020;2(10):1034-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schertzer JD, Tamrakar AK, Magalhães JG, et al. NOD1 activators link innate immunity to insulin resistance. Diabetes. 2011;60(9):2206-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan KL, Tam TH, Boroumand P, et al. Circulating NOD1 activators and hematopoietic NOD1 contribute to metabolic inflammation and insulin resistance. Cell Rep. 2017;18(10):2415-2426. [DOI] [PubMed] [Google Scholar]
- 5. Sepehri Z, Kiani Z, Javadian F, et al. TLR3 and its roles in the pathogenesis of type 2 diabetes. Cell Mol Biol (Noisy-Le-Grand). 2015;61(3):46-50. [PubMed] [Google Scholar]
- 6. Sepehri Z, Kiani Z, Nasiri AA, Kohan F. Toll-like receptor 2 and type 2 diabetes. Cell Mol Biol Lett. 2016;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmad R, Kochumon S, Thomas R, Atizado V, Sindhu S. Increased adipose tissue expression of TLR8 in obese individuals with or without type-2 diabetes: significance in metabolic inflammation. J Inflamm (Lond). 2016;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh K, Agrawal NK, Gupta SK, Sinha P, Singh K. Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J Diabetes Complications. 2016;30(1):99-108. [DOI] [PubMed] [Google Scholar]
- 9. Gupta S, Maratha A, Siednienko J, et al. Analysis of inflammatory cytokine and TLR expression levels in type 2 diabetes with complications. Sci Rep. 2017;7(1):7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363-1377.e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denou E, Lolmède K, Garidou L, et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7(3):259-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cavallari JF, Fullerton MD, Duggan BM, et al. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 2017;25:1063-1074.e1063. [DOI] [PubMed] [Google Scholar]
- 13. Cavallari JF, Pokrajac NT, Zlitni S, Foley KP, Henriksbo BD, Schertzer JD. NOD2 in hepatocytes engages a liver-gut axis to protect against steatosis, fibrosis, and gut dysbiosis during fatty liver disease in mice. Am J Physiol Endocrinol Metab. 2020;319(2):E305-E314. [DOI] [PubMed] [Google Scholar]
- 14. Cavallari JF, Barra NG, Foley KP, et al. Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice. Am J Physiol Endocrinol Metab. 2020;318(4):E579-E585. [DOI] [PubMed] [Google Scholar]
- 15. Al-Daghri NM, Clerici M, Al-Attas O, et al. A nonsense polymorphism (R392X) in TLR5 protects from obesity but predisposes to diabetes. J Immunol. 2013;190(7):3716-3720. [DOI] [PubMed] [Google Scholar]
- 16. Center for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 17. Sarwar N, Gao P, Seshasai SR, et al. ; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association. Standards of medical care in diabetes: 2018 abridged for primary care providers. Clin Diabetes. 2018;36:14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuschieri S. The genetic side of type 2 diabetes: a review. Diabetes Metab Syndr. 2019;13(4):2503-2506. [DOI] [PubMed] [Google Scholar]
- 20. Nasykhova YA, Barbitoff YA, Serebryakova EA, Katserov DS, Glotov AS. Recent advances and perspectives in next generation sequencing application to the genetic research of type 2 diabetes. World J Diabetes. 2019;10(7):376-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doumatey AP, Ekoru K, Adeyemo A, Rotimi CN. Genetic basis of obesity and type 2 diabetes in Africans: impact on precision medicine. Curr Diab Rep. 2019;19(10):105. [DOI] [PubMed] [Google Scholar]
- 22. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151-S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran HQ, Ley RE, Gewirtz AT, Chassaing B. Flagellin-elicited adaptive immunity suppresses flagellated microbiota and vaccinates against chronic inflammatory diseases. Nat Commun. 2019;10(1):5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luck H, Khan S, Kim JH, et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat Commun. 2019;10(1):3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312-11319. [DOI] [PubMed] [Google Scholar]
- 27. Xiong Y, Miyamoto N, Shibata K, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A. 2004;101(4):1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767-16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bjursell M, Admyre T, Göransson M, et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2011;300(1):E211-E220. [DOI] [PubMed] [Google Scholar]
- 30. Kimura I, Inoue D, Maeda T, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A. 2011;108(19):8030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell Physiol Biochem. 2011;28(5):949-958. [DOI] [PubMed] [Google Scholar]
- 32. Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimura I, Inoue D, Hirano K, Tsujimoto G. The SCFA receptor GPR43 and energy metabolism. Front Endocrinol (Lausanne). 2014;5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue D, Tsujimoto G, Kimura I. Regulation of energy homeostasis by GPR41. Front Endocrinol (Lausanne). 2014;5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou D, Chen YW, Zhao ZH, et al. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50(12):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen G, Huang B, Fu S, et al. G protein-coupled receptor 109A and host microbiota modulate intestinal epithelial integrity during sepsis. Front Immunol. 2018;9:2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong YH, Nishimura Y, Hishikawa D, et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146(12):5092-5099. [DOI] [PubMed] [Google Scholar]
- 41. Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation: protective or causative? Front Immunol. 2016;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fleischer J, Bumbalo R, Bautze V, Strotmann J, Breer H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res. 2015;361(3):697-710. [DOI] [PubMed] [Google Scholar]
- 43. Wright RS, Anderson JW, Bridges SR. Propionate inhibits hepatocyte lipid synthesis. Proc Soc Exp Biol Med. 1990;195(1):26-29. [DOI] [PubMed] [Google Scholar]
- 44. Nishina PM, Freedland RA. Effects of propionate on lipid biosynthesis in isolated rat hepatocytes. J Nutr. 1990;120(7):668-673. [DOI] [PubMed] [Google Scholar]
- 45. Sahuri-Arisoylu M, Brody LP, Parkinson JR, et al. Reprogramming of hepatic fat accumulation and “browning” of adipose tissue by the short-chain fatty acid acetate. Int J Obes (Lond). 2016;40(6):955-963. [DOI] [PubMed] [Google Scholar]
- 46. Jin CJ, Sellmann C, Engstler AJ, Ziegenhardt D, Bergheim I. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr. 2015;114(11):1745-1755. [DOI] [PubMed] [Google Scholar]
- 47. Zhou D, Pan Q, Xin FZ, et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23(1):60-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chambers ES, Viardot A, Psichas A, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64(11):1744-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261-273. [DOI] [PubMed] [Google Scholar]
- 50. Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787-1794. [DOI] [PubMed] [Google Scholar]
- 51. Guo C-J, Allen BM, Hiam KJ, et al. Depletion of microbiome-derived molecules in the host using Clostridium genetics. Science. 2019;366:eaav1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heimann E, Nyman M, Pålbrink AK, Lindkvist-Petersson K, Degerman E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte. 2016;5(4):359-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frommherz L, Bub A, Hummel E, et al. Age-related changes of plasma bile acid concentrations in healthy adults–results from the cross-sectional KarMeN study. Plos One. 2016;11(4):e0153959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eggert T, Bakonyi D, Hummel W. Enzymatic routes for the synthesis of ursodeoxycholic acid. J Biotechnol. 2014;191:11-21. [DOI] [PubMed] [Google Scholar]
- 55. Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling — mechanisms and research needs. Nat Rev Endocrinol. 2019;15:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25(10):2020-2030. [DOI] [PubMed] [Google Scholar]
- 58. Bozadjieva N, Heppner KM, Seeley RJ. Targeting FXR and FGF19 to treat metabolic diseases-lessons learned from bariatric surgery. Diabetes. 2018;67(9):1720-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98(6):3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pols TWH, Puchner T, Korkmaz HI, Vos M, Soeters MR, de Vries CJM. Lithocholic acid controls adaptive immune responses by inhibition of Th1 activation through the vitamin D receptor. PLoS One. 2017;12(5):e0176715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714-719. [DOI] [PubMed] [Google Scholar]
- 63. Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435-9440. [DOI] [PubMed] [Google Scholar]
- 64. Kuhre RE, Wewer Albrechtsen NJ, Larsen O, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386-390. [DOI] [PubMed] [Google Scholar]
- 66. Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perino A, Pols TW, Nomura M, Stein S, Pellicciari R, Schoonjans K. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J Clin Invest. 2014;124(12):5424-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484-489. [DOI] [PubMed] [Google Scholar]
- 69. Choucair I, Nemet I, Li L, et al. Quantification of bile acids: a mass spectrometry platform for studying gut microbe connection to metabolic diseases. J Lipid Res. 2020;61(2):159-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vyhlídalová B, Bartoňková I, Jiskrová E, Li H, Mani S, Dvořák Z. Differential activation of human pregnane X receptor PXR by isomeric mono-methylated indoles in intestinal and hepatic in vitro models. Toxicol Lett. 2020;324:104-110. [DOI] [PubMed] [Google Scholar]
- 71. Dong H, Lin W, Wu J, Chen T. Flavonoids activate pregnane x receptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC Biochem. 2010;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111(16):1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jin UH, Lee SO, Sridharan G, et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jin UH, Park H, Li X, et al. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicol Sci. 2018;164(1):205-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J Immunol. 2018;201(12): 3683-3693. [DOI] [PubMed] [Google Scholar]
- 76. Cason CA, Dolan KT, Sharma G, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018;68:1552-1562.e1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murphy N, Achaintre D, Zamora-Ros R, et al. A prospective evaluation of plasma polyphenol levels and colon cancer risk. Int J Cancer. 2018;143(7):1620-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Natividad JM, Agus A, Planchais J, et al. Impaired Aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018;28:737-749.e734. [DOI] [PubMed] [Google Scholar]
- 79. de Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tuomainen M, Lindström J, Lehtonen M, et al. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr Diabetes. 2018;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cussotto S, Delgado I, Anesi A, et al. Tryptophan metabolic pathways are altered in obesity and are associated with systemic inflammation. Front Immunol. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. J Neurogastroenterol Motil. 2018;30:e13178. [DOI] [PubMed] [Google Scholar]
- 83. Abbas M, Saeed F, Anjum FM, et al. Natural polyphenols: an overview. Int J Food Prop. 2017;20:1689-1699. [Google Scholar]
- 84. Murota K, Nakamura Y, Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci Biotechnol Biochem. 2018;82(4):600-610. [DOI] [PubMed] [Google Scholar]
- 85. Poesen R, Claes K, Evenepoel P, et al. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol. 2016;27(11):3479-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nemet I, Saha PP, Gupta N, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180:862-877.e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Koh A, Molinaro A, Ståhlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175:947-961.e917. [DOI] [PubMed] [Google Scholar]
- 88. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wallrabenstein I, Kuklan J, Weber L, et al. Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS One. 2013;8(2):e54950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Warrier M, Shih DM, Burrows AC, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10(3):326-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Miao J, Ling AV, Manthena PV, et al. ; Morbid Obesity Study Group . Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schugar RC, Shih DM, Warrier M, et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017;19(12):2451-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476-481. [DOI] [PubMed] [Google Scholar]
- 97. Chen YM, Liu Y, Zhou RF, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511-12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135(17):1671-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhu W, Buffa JA, Wang Z, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16(9):1857-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pathak P, Helsley RN, Brown AL, et al. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am J Physiol Heart Circ Physiol. 2020;318(6):H1474-H1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24(9):1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen S, Henderson A, Petriello MC, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30:1141-1151.e1145. [DOI] [PubMed] [Google Scholar]
- 106. Obeid R. Trimethylamine N-oxide and platelets aggregation: insufficient evidence for causal inference in thrombosis. AME Med J. 2017;2. [Google Scholar]
- 107. Jia J, Dou P, Gao M, et al. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional mendelian randomization analysis. Diabetes. 2019;68(9):1747-1755. [DOI] [PubMed] [Google Scholar]
- 108. Fernandez-Garcia JC, Delpino-Rius A, Samarra I, et al. Type 2 diabetes is associated with a different pattern of serum polyamines: a case-control study from the PREDIMED-plus trial. J Clin Med. 2019;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Colosimo DA, Kohn JA, Luo PM, et al. Mapping interactions of microbial metabolites with human G-protein-coupled receptors. Cell Host Microbe. 2019;26:273- 282.e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mastracci TL, Robertson MA, Mirmira RG, Anderson RM. Polyamine biosynthesis is critical for growth and differentiation of the pancreas. Sci Rep. 2015;5:13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pryor R, Norvaisas P, Marinos G, et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of metformin therapy. Cell. 2019;178:1299-1312.e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Deng A, Munger KA, Valdivielso JM, et al. Increased expression of ornithine decarboxylase in distal tubules of early diabetic rat kidneys: are polyamines paracrine hypertrophic factors? Diabetes. 2003;52(5):1235-1239. [DOI] [PubMed] [Google Scholar]
- 113. Cohen LJ, Esterhazy D, Kim SH, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549(7670):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153(Suppl 1):S76-S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Li NX, Brown S, Kowalski T, et al. GPR119 agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes. 2018;67(7):1401-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cohen LJ, Kang HS, Chu J, et al. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist. Proc Natl Acad Sci U S A. 2015;112(35):E4825-E4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tan X, Liu Y, Long J, et al. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res. 2019;63:1900257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.