Fig. 5.
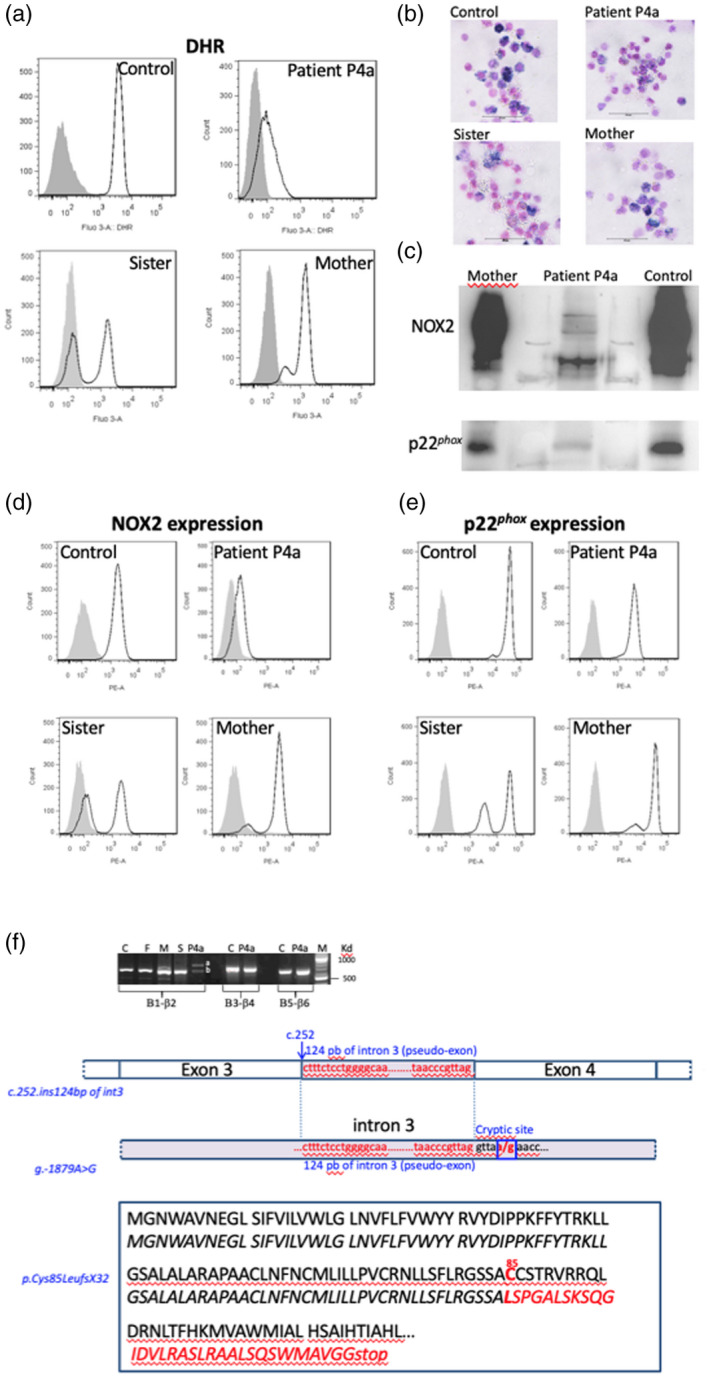
Phenotypical analysis of the neutrophils of patient P4a suffering from an X91−‐chronic granulomatous disease (CGD) and having a new CYBB mutation. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) oxidase activity of neutrophils from patient P4a, his sister and his mother was measured by flow cytometry after stimulation during 15 min with 200 ng/ml phorbol myristate acetate (PMA) in the presence of the dihydrorodamine (DHR) probe, as described in Materials and methods. Solid grey curve corresponds to DHR‐loaded resting neutrophils, empty black curve corresponds to DHR‐loaded neutrophils after PMA stimulation (a). The NADPH oxidase activity of neutrophils from patient P4a, his sister and his mother was also measured using the nitro blue‐tetrazolium (NBT) reduction test (b). NOX2 and p22phox expression was shown by Western blot using the primary specific antibodies 48 and 449, respectively (c). Nox2 (d) and p22phox expression (e) was shown by flow cytometry using the primary specific antibodies 7D5 and 449, respectively. Solid grey curve corresponds to resting neutrophils labeled with irrelevant antibodies; empty black curve corresponds to neutrophils labeled with the 7D5 (anti‐NOX2) or 449 antibodies (p22phox) and secondary antibodies coupled with phycoerythrin (PE). Genetic analysis of the mutation of patient P4a in CYBB gene, leading to an X91−‐CGD. NOX2 cDNA was amplified in three overlapping fragments, as described in Materials and methods. A 124 base pair (bp) of intron 3 was inserted between exons 3 and 4 leading to the creation of a pseudoexon. Intronic regions surrounding the inserted region of intron 3 in the NOX2 cDNA was amplified and sequenced. A c.253‐1879A>G hemizygous mutation was found creating a splicing donor site, which unveils a cryptic acceptor site leading the inclusion of 124 nucleotides as a pseudo‐exon responsible for the partial loss of the NOX2 expression. The consequence in the NOX2 protein is a missense mutation at Cys85→Leu and a frameshift leading to the generation of a stop codon located 32 amino acids further on (p.Cys85LeufsX32) (f).
