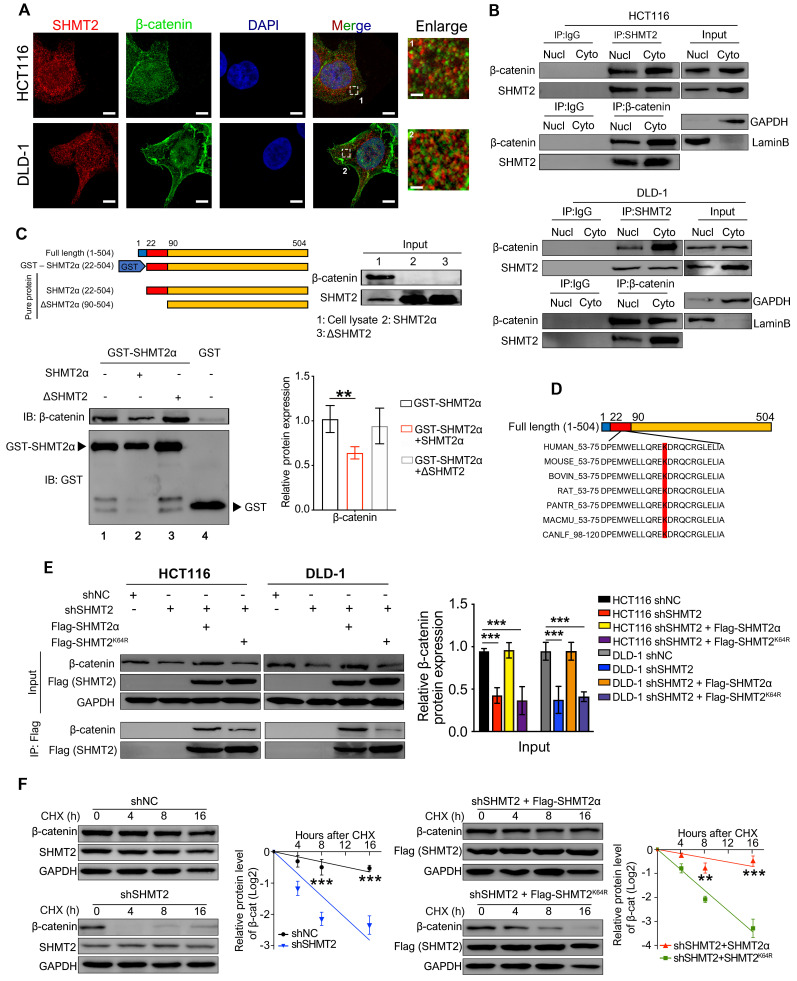
Figure 5.
SHMT2 interacts with β-catenin at K64. (A) Representative immunofluorescence images of SHMT2 (blue) and β-catenin (red) in HCT116 and DLD-1 cells. Scale bars, 5 μm, 1 μm. (B) The cytoplasmic and nuclear protein was separated from HCT116 and DLD-1 cells and subjected to immunoprecipitation using the indicated antibodies. (C) Pure SHMT2 protein with the different indicated constructions were added into the cell lysates derived from HCT116 cells. Immunoblot analysis of GST pull-down using the indicated antibodies. The relative β-catenin expression (normalized to GST-SHMT2α) was quantified. ** p < 0.01; Student's t-test. (D) The alignment of SHMT2 for human, mouse, bovin, rat, pantr, macmu, and canlf, shows highly conserved residues in SHMT2, including lysine 64. (E) Stable SHMT2 knockdown HCT116 and DLD-1 cells that re-expressed the shRNA-resistant SHMT2α or K64R mutation were constructed. The cells were subjected to immunoprecipitation using the indicated antibodies. (F) Western blot analysis (left) and quantification (right) of β-catenin in HCT116 cells with stable SHMT2 knockdown that re-expressed shRNA-resistant SHMT2α or K64R mutation after cycloheximide (CHX) treatment at different time points. ** p < 0.01, *** p < 0.001; Student's t-test. The data are presented as the mean ± SD from at least three independent experiments. The error bars show the SD.