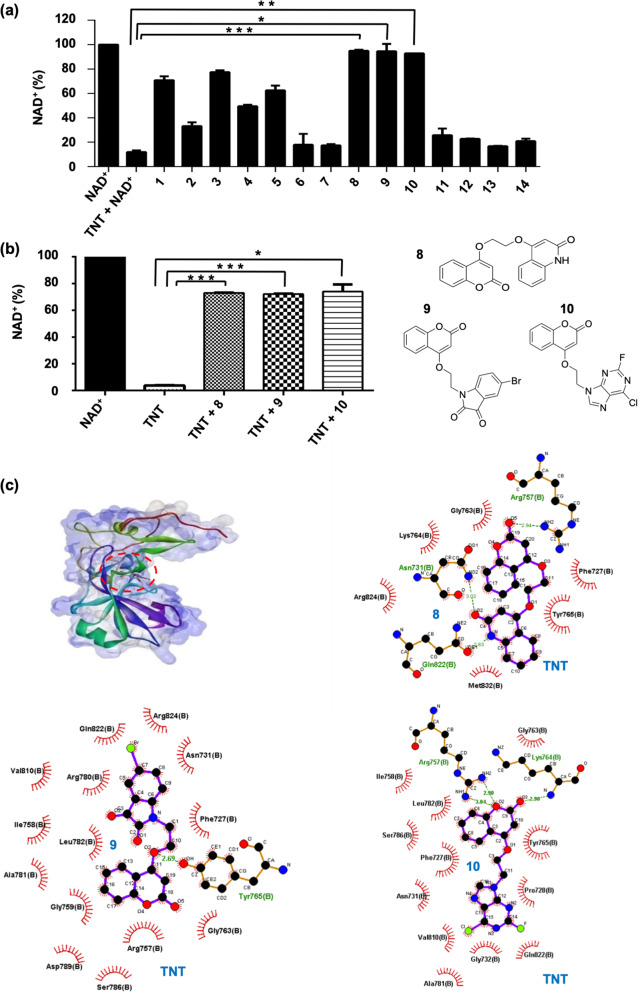
Fig. 6. Evaluation of inhibitors against NAD+-glycohydrolase activity of TNT.
a Determination of inhibitory potential of small molecules against NAD+-glycohydrolase activity of TNT using NADH fluorescence assay. 10 µM of small-molecule inhibitors was incubated with 75 nM of TNT followed by addition of 100 µM NAD+ to initiate the reaction. The remaining NAD+ levels were then measured. NAD+ level of control reaction without TNT was considered as 100%. Percent decrease in NAD+ content represents NAD+-glycohydrolase activity. b Inhibition of NAD+-glycohydrolase activity of TNT by selected molecules 8, 9, and 10 using enzyme-coupling assay. Recombinant TNT (30 nM) was incubated with 1 µM of inhibitor followed by addition of 5 µM NAD+. NAD+-glycohydrolase activity of TNT was measured using enzyme coupling assay. The P values were calculated using unpaired t-test with Welch’s correction. *P < 0.05, **P < 0.01, ***P < 0.001. Statistical significance was determined considering TNT treated sample as the control against which other groups were compared. c 3D surface model of TNT-8, TNT-9, and TNT-10 complex. Ligplot analysis of these interactions and Hydrogen-bonding analysis of TNT-8, TNT-9, and TNT-10 complex showing minimum binding energy of −8.92 kcal/mol, −8.43 kcal/mol, and −7.33 kcal/mol, respectively.