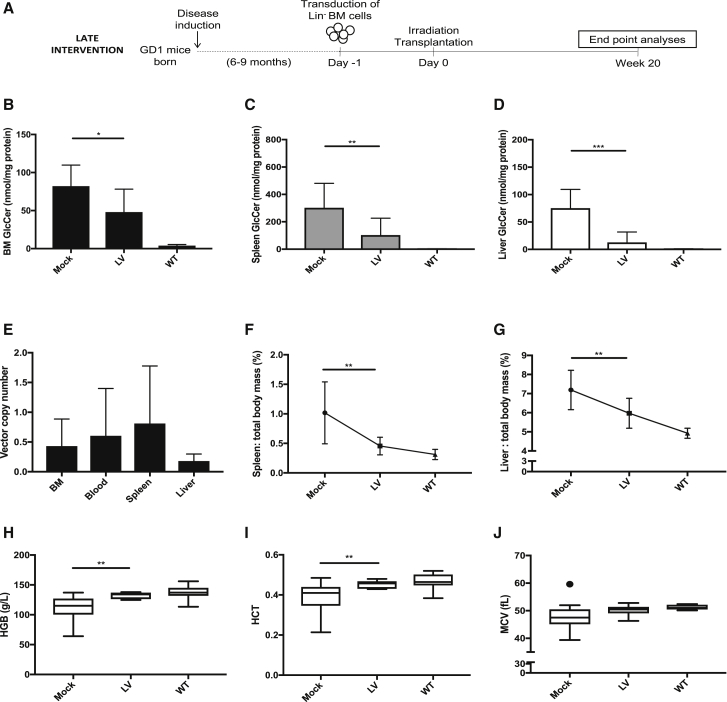
Figure 3.
Robust reduction of established Gaucher disease pathology by lentiviral gene therapy
(A) Experimental design of the late intervention study. (B–D) GlcCer quantification in BM (B), spleen (C), and liver (D) 20 weeks posttransplant. (E) VCN was estimated in whole tissue lysates from BM, blood, spleen, and liver samples. (F) Relative spleen mass reduction (graph depicting the percentage of spleen mass: total body mass for individual mice). (G) Relative liver mass reduction (graph depicting the percentage of liver mass: total body mass for individual mice). (H–J) Blood samples analyzed for hemoglobin (HGB) (H), hematocrit (HCT) (I), and mean red blood cell volume (MCV) (J). Mock, n = 15; LV, n = 10; WT, n = 8. Mann-Whitney U-test; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001. Error bars represent mean ± SD.