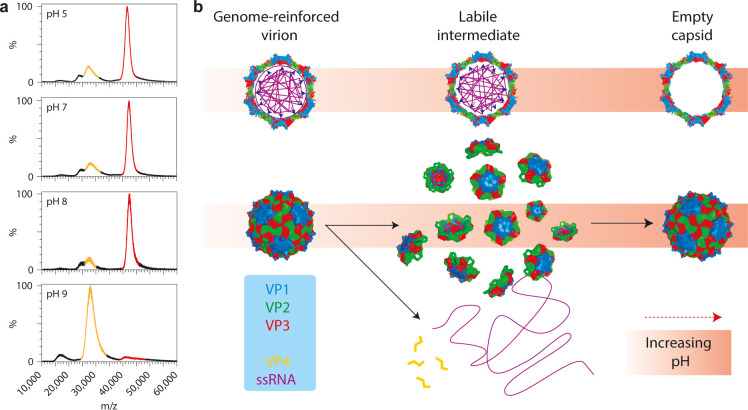
Figure 5.
Alkaline-triggered uncoating of Triatoma virus (TrV) monitored by native mass spectrometry. (a) Spectra of TrV virions, incubated at different pHs. Signal corresponding to virion is highlighted in red (m/z ∼40000; Mw ∼8.3 MDa), that of empty capsids in yellow (m/z ∼28000; Mw ∼5.4 MDa). (b) Model of the alkaline-triggered uncoating of TrV. Under neutral pH, TrV confines a very large genome. This comes at a high energetic cost, but the ssRNA stabilizes the capsid, thereby preventing premature uncoating. At higher pH, this stabilizing interaction is lost and electrostatic self-repulsion of the ssRNA increases due to loss of charge on counterions; the capsid bursts and falls apart into pentons. The genome and VP4 are released into solution and pentons reassemble into empty capsids. Adapted by permission from Macmillan Publishers Ltd.: Nat. Chem. Snijder, J.; Uetrecht, C.; Rose, R. J.; Sanchez-Eugenia, R.; Marti, G. A. et al. 2013 Probing the biophysical interplay between a viral genome and its capsid. Nat. Chem.5(6): 502–509 (ref (24)). Copyright 2013.