Figure 7.
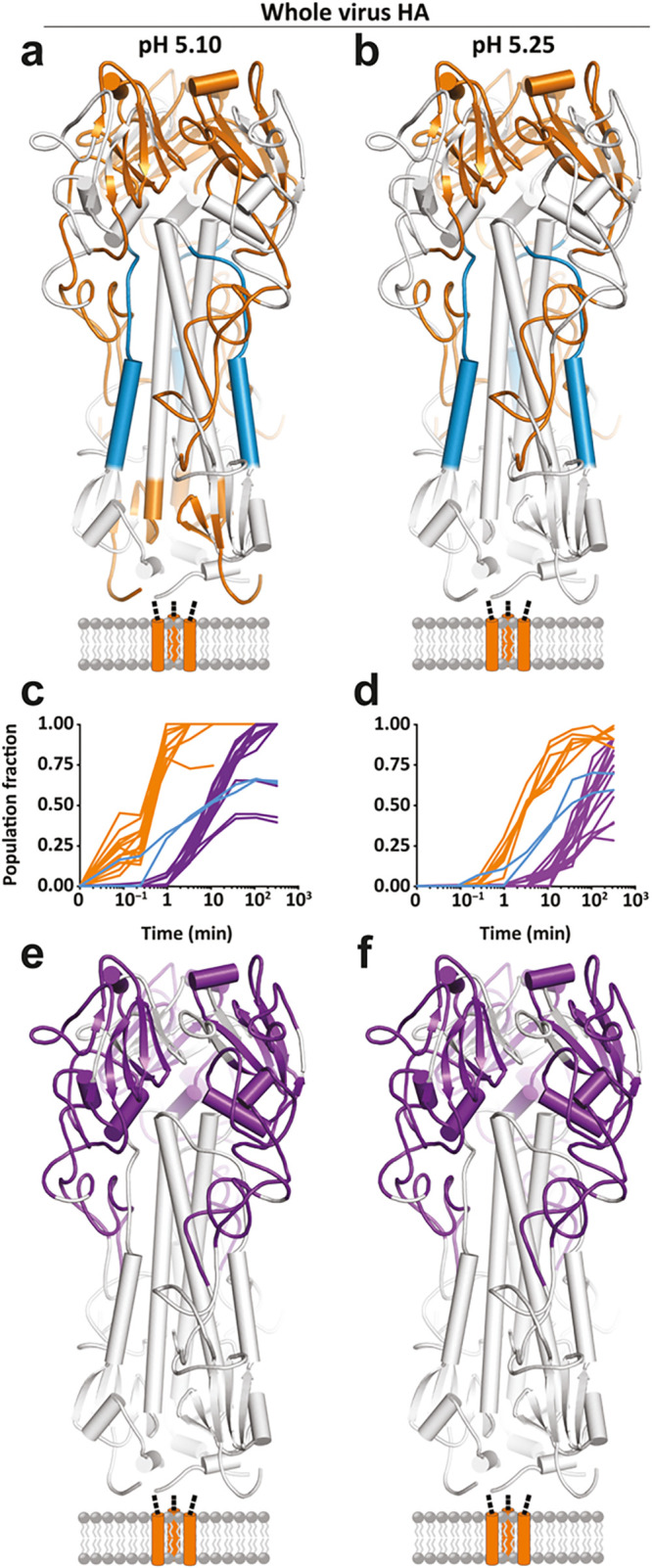
Global kinetic comparison and pH dependence of the fusion activation in influenza hemagglutinin (HA) investigated by HDX-MS. HDX-MS of full-length HA embedded in the whole virion is used to monitor the pH triggered refolding from the pre- to postfusion state. The specific pH of activation did not alter the nature (a and b) or sequence of any observed structural changes in full-length HA but simply accelerated the onset and rate of change of each conformational event (c and d). Conformational transitions that take place before 3.5 min are colored in orange. The last transition reported by the HA1 peptides is to a largely unfolded, highly flexible state, which begins at 3.5 min [purple; (c and d) and (e and f)]. Formation of the postfusion helical bundle in full-length HA is delayed because of the formation of the intermediate state [(a–d); blue peptides and traces]. Reprinted from Benhaim, M. A.; Mangala Prasad, V.; Garcia, N. K.; Guttman, M.; Lee, K. K. 2020 Structural monitoring of a transient intermediate in the hemagglutinin fusion machinery on influenza virions. Sci. Adv.6(18): eaaz8822 (ref (73)). Copyright The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. Distributed under a Creative Commons Attribution Non-Commercial License 4.0 (CC BY-NC) http://creativecommons.org/licenses/by-nc/4.0/.
