Learning objectives.
By reading this article, you should be able to:
-
•
Describe the physiological changes that occur during surgery.
-
•
Discuss how a method of anaesthesia or analgesia may be chosen, based on its effect on the stress response.
-
•
Explain the role of the anaesthetist in attenuating the surgical stress response in conjunction with surgical and other multidisciplinary colleagues.
Key points.
-
•
The stress response to surgery consists of two main components: neuroendocrine–metabolic and inflammatory–immune.
-
•
After surgery, there is a state of hyper-catabolism, which produces readily useable metabolic energy sources.
-
•
Cytokine production is related to the degree of surgical tissue injury.
-
•
Inhibition of the stress response is greatest with central neural blockade and minimally invasive surgery.
-
•
General anaesthesia has little effect on cytokine responses as it cannot influence direct tissue trauma.
The stress response to surgery is a pattern of physiological and pathophysiological changes that occur in response to the stimulus of surgery. The response consists of two broad categories:
-
(i)
neuroendocrine–metabolic response
-
(ii)
inflammatory–immune response
The magnitude, invasiveness, and duration of surgery are central in determining the degree of the body's integrated stress response. Major open vascular and abdominal surgery, joint replacement surgery, and cardiac surgery using cardiopulmonary bypass (CPB) elicit the greatest stress response.1,2 The body's attempt to maintain physiological homeostasis perioperatively induces a non-specific adaptation response that can be detrimental and lead to systemic inflammatory response syndrome (SIRS), hyper-metabolism, and hyper-catabolism. Further injury may cause loss of normally functioning negative-feedback systems leading to muscle wasting, impaired immune function, impaired wound healing, and the potential for organ failure and death.
Physiological responses to surgical stress
Neuroendocrine–metabolic response
Sympathetic nervous system response
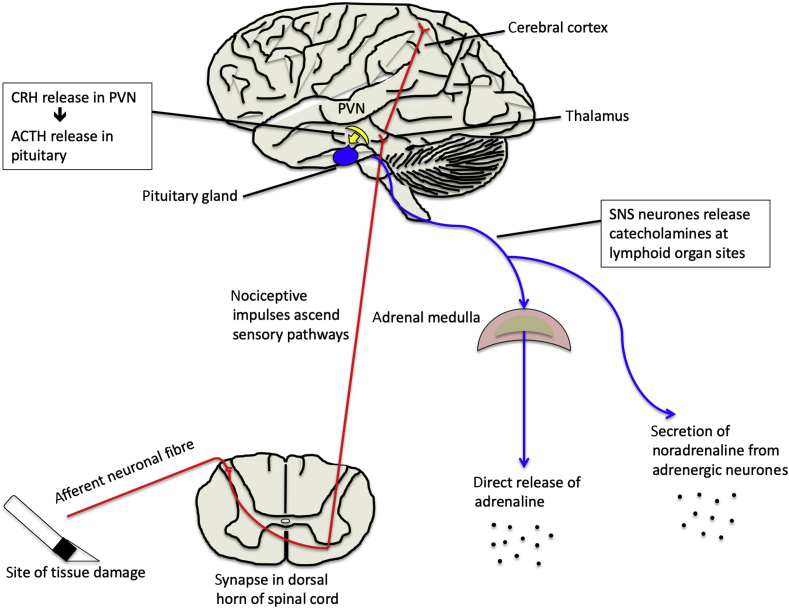
The paraventricular nucleus (PVN) is a group of neurones in the hypothalamus that plays a central role during stress. The PVN can detect physiological changes, such as hypotension and inflammation. It acts to relay afferent impulses originating at the site of surgical tissue damage via the limbic system, particularly the amygdala and brainstem nuclei (Fig 1). Paraventricular nucleus fibres project directly to the posterior pituitary and also control various anterior pituitary functions. Adrenaline (epinephrine) is secreted directly from the adrenal medulla in response to hypothalamic activation by the sympathetic nervous system (SNS) (Fig 1). Circulating adrenaline strengthens the sympathetic response and mobilises carbohydrate and fat stores. Rapid sympathetic responses are mediated neurally. However, slower sustained sympathetic responses are also seen, and these result from hormonal responses (i.e. circulating adrenaline and noradrenaline [norepinephrine]).3
Fig 1.
Hypothalamic activation of the neuroendocrine response.
Heart rate and vascular smooth muscle tone are controlled by the SNS. Sympathetic nervous system activation increases efferent signals to vascular smooth muscle, thereby increasing systemic vascular resistance and arterial blood pressure. Blood flow to active muscles is increased alongside a concurrent reduction in blood flow to organs not prioritised for rapid motor activity, such as the kidneys and gastrointestinal tract. Hepatic and muscle lipolysis and glycogenolysis increase, leading to hyperglycaemia. In addition, cellular metabolic activity and the coagulability of blood increase.
Endocrine system response
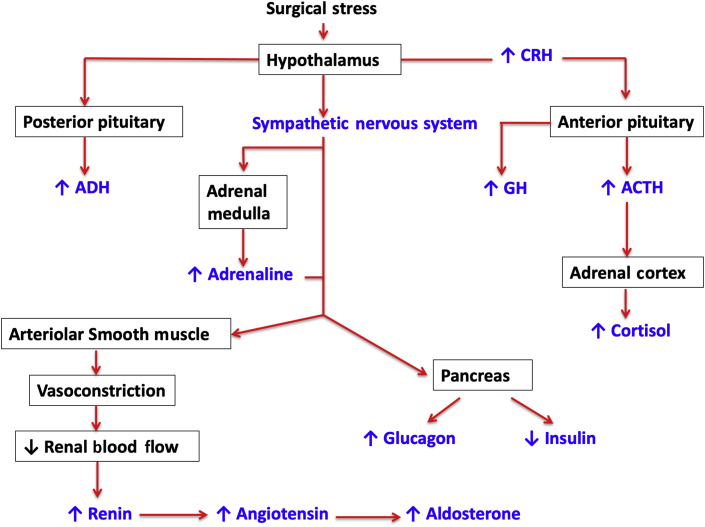
The hypothalamus both directly and indirectly coordinates the complex hormonal stress response (Fig 2). Corticotrophin-releasing hormone (CRH), secreted in response to surgical stress, activates the hypothalamic–pituitary–adrenal (HPA) axis cascade and its metabolic consequences. Corticotrophin-releasing hormone stimulates the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH). Adrenocorticotropic hormone acts on cells in the zona fasciculata of the adrenal cortex to promote glucocorticoid (cortisol) secretion.
Fig 2.
Integration of the stress response by the hypothalamus, sympathoadrenal, and sympathorenal responses.
In the normal ‘unstressed’ state, physiological levels of glucocorticoid hormones participate in conventional negative-feedback mechanisms to inhibit ACTH and CRH secretion, predominantly at the level of the anterior pituitary gland, but also at the PVN. Activity of the HPA axis is characterised by a circadian rhythm with superimposed ultradian pulsatile release of glucocorticoids (i.e. there is a recurrent cycle of release repeated throughout a 24 h period). The circadian pattern of cortisol release is controlled by the suprachiasmatic nucleus in the hypothalamus. The HPA axis is a stress-responsive neuroendocrine system that adapts and responds to homeostatic challenges, such as surgery. Immediately after surgery, ultradian pulses in ACTH and cortisol both increase.4 Adrenocorticotropic hormone concentrations return to baseline within 24 h, but plasma concentrations of cortisol remain increased for at least 7 days after major surgery. In minimally invasive surgical procedures, when compared with open surgical techniques, the cortisol 'peak' is delayed, and in severe critical illness, the circadian variation is flattened in proportion to the degree of circadian disruption.5
Chronic activation of the HPA axis before surgery is associated with HPA axis dysfunction. Increasing age and frailty are associated with a progressive loss of hypothalamic sensitivity, with higher cortisol concentrations and a decrease in its diurnal variation. Negative-feedback mechanisms of the HPA axis are blunted: CRH (and also vasopressin, discussed in more detail later) in the PVN of elderly people is increased despite increased circulating plasma cortisol concentrations. Pre-existing cardiovascular deconditioning leading to decreased physical activity, as a result of conditions, such as heart failure, chemotherapy for cancer, or chronic joint pain, can contribute to perioperative disruption of neuroendocrine function.6 Hypothalamic–pituitary–adrenal axis dysfunction also occurs in a variety of non-cardiac medical conditions, including clinical depression; anxiety and depression are associated with worse perioperative outcomes. Central hypersecretion of CRH, and consequently increased production of glucocorticoids, may contribute to the HPA axis dysregulation that occurs in 80% of patients with depression. This may partly account for the increased incidence of upper respiratory tract infections, disruption in wound healing, and psychosocial stress when these patients undergo surgery.5
Growth hormone (GH) secretion by the anterior pituitary gland increases in response to the magnitude of the surgical stress response (Fig 2). Growth hormone increases hepatic glycogenolysis leading to hyperglycaemia. Growth hormone also causes insulin resistance, although the molecular mechanism is uncertain.
Antidiuretic hormone (ADH) is a peptide hormone that is synthesised in the hypothalamus before being transported via axons to the posterior pituitary gland and released into the circulation (Fig 2). This process occurs in response to hypovolaemia, hypotension, hyperosmolarity, and an increase in angiotensin II concentrations. The primary function of ADH, also termed vasopressin or arginine vasopressin, is to regulate extracellular fluid volume. Antidiuretic hormone release leads to a reduction in renal free-water clearance by an action on the renal collecting ducts. Antidiuretic hormone stimulates the insertion of aquaporins into the walls of the renal collecting system. This favours free-water resorption down its concentration gradient back into the renal medulla and causes a reduction in urine volume with an increase in urine concentration.
Other hormonal changes associated with the stress response include increased prolactin concentrations and reduced testosterone, thyroxine (T4), and triiodothyronine (T3) concentrations. These normalise to preoperative baseline within a few days and are not thought to exert a significant effect on patient-centred functional outcomes.
Metabolic response
A state of hypermetabolism and hypercatabolism occurs with the mobilisation of readily useable energy sources (Table 1). Hepatic glycogen stores are converted to glucose, skeletal muscle undergoes proteolysis, and fat reserves undergo lipolysis. The body uses these substrates in tissue repair and as an energy source.
Table 1.
Summary of catabolic fuel metabolism
| Metabolic process | Catabolic reaction | Caused by | Effect |
|---|---|---|---|
| Hepatic gluconeogenesis | Amino acids → glucose | Increased adrenaline, glucagon, and cortisol concentrations stimulate this mobilisation of fuel stores | Increased blood glucose Protein catabolism |
| Hepatic glycogenolysis | Glycogen → glucose | Increased blood glucose | |
| Lipolysis | Triglycerides → fatty acids and glycerol | Increased plasma fatty acids | |
| Proteolysis | Protein → amino acids | Increased plasma amino acids |
The release of adrenaline seen alongside SNS activation results in the stimulation of glucagon and inhibition of insulin release. Secretion of the key anabolic hormone insulin is reduced by the SNS effect on pancreatic α2-adrenergic receptors, and later a decrease in insulin sensitivity occurs in peripheral cells. These hormonal changes lead to hyperglycaemia and the release of fatty acids with relatively unopposed catabolism of muscle tissue.3
Increased sympathetic activity to the kidneys activates the renin–angiotensin–aldosterone system (RAAS). Adrenaline-induced vasoconstriction of the renal afferent arterioles causes reduced renal blood flow, promoting the secretion of renin. Renin initiates conversion of angiotensin I to angiotensin II by angiotensinogen, which in turn stimulates the release of aldosterone from the adrenal cortex. In addition, the posterior pituitary gland secretes ADH in response to both increased sympathetic activity and angiotensin II. Together, the hormones aldosterone and ADH promote the retention of salt and water. These changes play a role in the sustained maintenance of blood volume and increased vascular tone. Fluid retention, oliguria, and accumulation of extracellular fluid are common in the acute postoperative period. This may act as a protective feature to help maintain arterial blood pressure in the setting of acute loss of plasma volume through, for example, haemorrhage. In addition, ADH and angiotensin have a direct vasopressor effect.3
Inflammatory–immune response
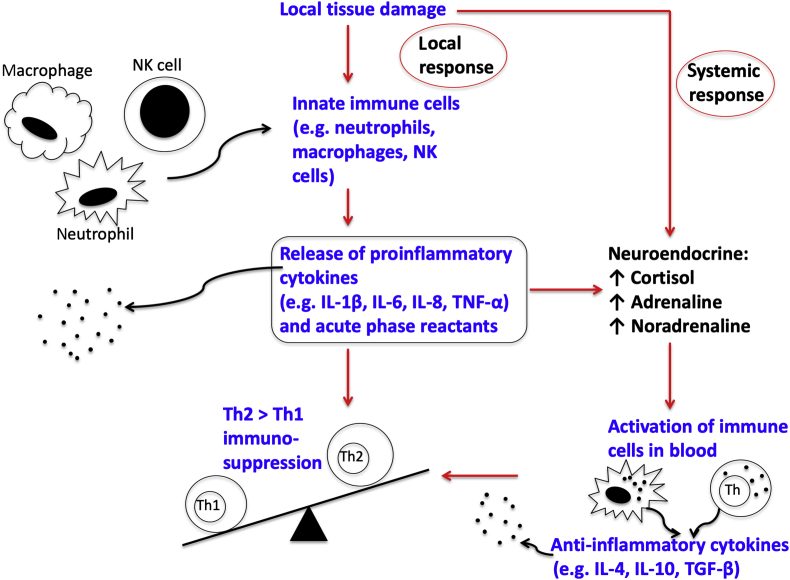
The stress response to surgery involves both the innate and cell-mediated adaptive (acquired) immune systems. In addition to the magnitude of surgical trauma, factors, such as malnutrition, infection, and cancer status, contribute to the impact of surgery on the inflammatory–immune system. The innate immune response is non-specific, and activation occurs early in the surgical stress response. Monocytes migrate to sites of tissue injury where they differentiate into macrophages. Innate immune cells with phagocytic properties capable of antigen presentation, such as macrophages, and neutrophils and natural killer (NK) cells, move into the wound and produce proinflammatory mediators called cytokines (Fig 3).7 If there is excessive production of inflammatory mediators, a SIRS response characterised by non-specific whole-body response may occur. Natural killer cells are a type of uncharacterised cytotoxic lymphocyte and an important component of the innate immune system. Natural killer cells are activated in response to cytokines and induce apoptosis in damaged, neoplastic, and virally infected cells. Later after operation, immune cells may have reduced cytotoxicity.8
Fig 3.
Surgery-induced immunological response. TGF, transforming growth factor.
Cytokines are a vast family of small proteins with diverse biological activity that include interleukins (ILs), interferons, chemokines, and tumour necrosis factors (TNFs). Cytokines are responsible for mediating and maintaining the local inflammatory response to tissue damage. Early in the inflammatory cascade, pro-inflammatory cytokines IL-6, IL-1β, TNF-α, and IL-8 are produced (Fig 3). Cytokine concentrations are highest on the first postoperative day. Anti-inflammatory cytokines IL-4, IL-10, transforming factor-β, IL-1 receptor antagonist, and soluble TNF receptors (sTNFR1 and sTNFR2) are also produced. They help to reduce the magnitude and duration of the SIRS response. If the overall balance between the pro- and anti-inflammatory mediated responses is unregulated, immunodeficiency and sepsis are more likely.9
The acute phase response is an increase in the concentration of serum proteins (acute phase proteins [APPs]) in response to tissue injury and inflammation (Fig 3), and is a component of early innate immunity. Acute phase proteins are a large group of proteins produced by hepatocytes in response to stimulation by cytokines, especially IL-6. The acute phase response is characterised by the production of APPs in the liver, such as C-reactive protein (CRP), fibrinogen, D-dimer, and α2 macroglobulin, and also fever, granulocytosis, and a reduction in transport proteins, such as albumin and transferrin.
Initiation of the cell-mediated adaptive immune response requires interaction between T-lymphocytes and antigen-presenting cells. Antigen-presenting cells, such as macrophages, take up antigens, such as damaged tissue cells in the surgical wound or tumour cells. The surgical stress response promotes a relative increase in T-helper 2 (Th2) lymphocytes compared with T-helper 1 (Th1) lymphocytes (Fig 3). The shift in Th1:Th2 balance causes impaired cell-mediated immunity, and this is associated with poor outcomes, such as impaired wound healing, an increased incidence of sepsis, and cancer recurrence; it can also contribute to the development of postoperative multi-organ failure.10 The suppression of Th1 response and an increase in Th2 response leading to impaired cell-mediated immunity may be detrimental after all major surgery. Furthermore, in cancer surgery, this shift may result in deficient NK cell function, and therefore higher risk of perioperative tumour cell immune evasion and possibly increasing the chance of cancer recurrence or metastasis.
Recovery after surgery has been tracked by serial measurement of a number of blood markers, such as cortisol, IL-6, white blood cell count (WCC), and CRP. The postoperative concentrations for these peak at different times: cortisol (0–4 h), IL-6 (12–24 h), WCC (24–48 h), and CRP (24–72 h). Only IL-6 and CRP concentrations are consistently associated with the magnitude of surgical stress. Peak cortisol concentrations and WCC are not considered to reflect the magnitude of systemic inflammatory response in elective surgery. C-reactive protein may be monitored after major surgery as a potential early indicator of infection or excessive inflammation.1
Modulation of the perioperative stress response
Anaesthetic drugs
I.V. and volatile anaesthetic agents
A single induction dose of propofol (1–2 mg kg−1) suppresses circulating cortisol concentrations, but does not completely block the secretion of cortisol and aldosterone. A continuous infusion of propofol (TIVA with plasma concentration of 4–8 μg ml−1) may completely block cortisol secretion.11
This inhibition of cortisol secretion is postulated to take place at the level of the adrenal glands because the ACTH response to surgery is similar during maintenance of anaesthesia with both propofol and volatile anaesthetics. Propofol results in the lowest proteolytic response to surgery, when compared with other i.v. and inhaled anaesthetic agents. This may be because the propofol emulsion contains triglycerides, which allows the body to preferentially use them as a substrate.12
Etomidate suppresses adrenocortical function by the reversible inhibition of 11β-hydroxylase and 17α-hydroxylase enzymes. Etomidate inhibits synthesis of cortisol and aldosterone for up to 8 h after a single induction dose (0.3 mg kg−1).9 Etomidate is considered obsolete because of its association with increased mortality when used as a sedative infusion in patients with sepsis in intensive care. Thiopental and ketamine have both been shown to suppress NK immune cell activity in in vitro animal models, but propofol has not.12
Volatile anaesthetic agents inhibit ACTH, cortisol, catecholamine, and GH to a greater extent than i.v. anaesthetic agents, such as propofol combined with remifentanil.12 In laparoscopic surgery, ACTH, cortisol, and GH concentrations are significantly reduced when sevoflurane is used compared with isoflurane.13 In cardiac surgery, there was no difference in mortality or other outcomes when volatile anaesthesia and TIVA with propofol were compared.14
Volatile agents impair platelet aggregation and clot stability to a greater extent than propofol.12 Volatile anaesthetic agents have a multitude of both immunosuppressive and immunoactivating effects. These immune-modulatory effects include decreased NK cell cytotoxicity by sevoflurane, isoflurane, and halothane; decreased cytokine release by sevoflurane; decreased neutrophil cell number and adhesion by sevoflurane, isoflurane, and halothane; and increased neutrophil cell number by desflurane.8
Analgesics and other medications
Benzodiazepines
Benzodiazepines (e.g. midazolam 0.2–0.4 mg kg−1 or infusion of 0.9–0.125 mg kg−1 h−1) inhibit cortisol production at the hypothalamic–pituitary level of the HPA axis. This effect has been reported in both limb and abdominal surgeries. The significance of this suppression is not clear.11
α2-adrenergic agonists
Clonidine and dexmedetomidine are centrally acting α2-adrenoceptor agonists that inhibit the surgical stress response mediated by the SNS. Central sympathetic outflow is reduced when α2-receptors in the lateral reticular nucleus are stimulated. In the spinal cord, α2-receptor stimulation augments endogenous opioid release and modulates the descending pathways involved in spinal nociceptive processing. By these mechanisms, the sympathoadrenal and cardiovascular responses to a surgical stimulus are reduced.
Dexmedetomidine reduces cortisol and renin concentrations, which imparts haemodynamic stability and impaired pancreatic insulin secretion. A single dose of dexmedetomidine 0.5 μg kg−1 before induction attenuates the increase in heart rate and MAP during laryngoscopy and tracheal intubation.15 After major abdominal surgery, a bolus of dexmedetomidine (0.5–1.0 μg kg−1) before induction followed by an infusion (0.2–0.5 μg kg−1 h−1) during the operation significantly suppressed postoperative IL-6, IL-8, TNF-α, cortisol, and glucose concentrations.16
Opioids
Systemic opioids reduce ACTH and GH secretion by reduced CRH release at the hypothalamic level. High-dose opioids have been shown to completely suppress both ACTH and cortisol secretion if administered before CPB in cardiac surgery (but not after CPB), and before knife to skin incision in open cholecystectomy.2,11 At high doses (fentanyl >50 μg kg−1), the hormonal response to pelvic and abdominal surgery is prevented.11 However, this dose level would significantly prolong re-emergence from anaesthesia and is associated with a requirement for postoperative ventilatory support.2 Systemic opioids may attenuate the hyperglycaemic response to surgery.
Morphine, fentanyl, remifentanil, methadone, and codeine have an immunomodulatory role, whereas oxycodone, tramadol, hydrocodone, and buprenorphine do not.17 One hypothesis to explain this is that opioids that can cross the blood–brain barrier (BBB) exert more of an immunomodulatory effect than opioids that cannot cross the BBB. Morphine shows dose-dependent, immunosuppressive effects to impair monocyte and neutrophil function, NK-cell-mediated cytotoxicity, cytokine release, and lymphocyte and macrophage proliferation.18 There is conflicting and inconsistent evidence in relation to the effect of opioids on tumour growth and cancer metastasis. Emerging preclinical literature in both in vitro and in vivo studies suggests that opioids may influence tumour cell growth by their action on the mu-opioid receptor (MOR). This receptor is overexpressed on the surface of certain cancers. In non-small cell lung cancer, the MOR antagonist methylnaltrexone may be beneficial in reducing cancer progression and metastasis.19 Similar to the relationship between opioids and cancer recurrence, the clinical consequences of the immunomodulation caused by high-dose opioids on perioperative infection rates are not yet fully understood.
Regional anaesthesia
Wide-ranging neuraxial analgesia with local anaesthetic agents block the endocrine and metabolic response to surgery in the pelvis and lower limbs. Much of the literature examining this does so as a comparative study with volatile or i.v. general anaesthesia agents. Neuraxial epidural and spinal anaesthesia block the HPA axis response by blocking the afferent activation of the hypothalamus and efferent stimulation of the liver, adrenals, and pancreas. Adrenocorticotropic hormone, cortisol, adrenaline, and GH secretions are impaired.12 Proposed benefits of regional techniques over general anaesthesia include an earlier return of gut function, reduced incidence of pulmonary dysfunction, reduced inflammatory response to surgery, and beneficial effects on the coagulation system.
In abdominal aortic surgery, epidural anaesthesia when combined with general anaesthesia reduces the increase in cortisol and urinary adrenaline concentrations during surgery, when compared with general anaesthesia alone.12 Thoracic epidural anaesthesia combined with general anaesthesia can suppress the catecholamine response during CPB and up to 24 h after surgery.2 In patients undergoing hip surgery, combined spinal and epidural blockade ( compared with general anaesthesia) reduced the amount of amino acid oxidation, as a marker of protein catabolism in the acute postoperative period.20 The hyperglycaemic response during surgery is inhibited by regional anaesthesia. Spinal anaesthesia showed suppression of serum cortisol and blood glucose concentrations compared with general anaesthesia in patients undergoing elective abdominal, urological, and orthopaedic surgery.21
Thoracic epidural combined with general anaesthesia can suppress the catecholamine response during CPB and up to 24 h after surgery.2
Surgical techniques
Minimally invasive surgical techniques, including laparoscopic and robotic surgeries, have an independent impact on the magnitude of the inflammatory–immune response. When used alongside enhanced recovery after surgery (ERAS) programmes, minimally invasive surgery can reduce hospital length of stay, surgical complication rates, and incidence of readmission to hospital.22
The duration of surgery and the extent of intraoperative surgical manipulation and tissue injury are proportional to the associated stress response. The use of less invasive surgical techniques over traditional open techniques where possible reduces the inflammatory response (e.g. concentrations of IL-6 and CRP are reduced in laparoscopic surgery when compared with open techniques). A major advantage of minimally invasive surgery is the reduced incision size and associated tissue injury, resulting in a reduction in requirements for postoperative analgesia.22
There is an increase in intra-abdominal pressure during minimally invasive pelvic and abdominal surgery with a pneumoperitoneum. This results in reduced renal blood flow with activation of the RAAS and an increase in ADH secretion. Permissive oliguria of 0.3 ml kg−1 h−1 and increased ADH secretion may be accepted as an appropriate physiological response that is not associated with an increased incidence of acute kidney injury.22
Glucocorticoids
The impact on the surgical stress response by the perioperative supplementation of glucocorticoids is an uncertain area, and there is significant heterogeneity between studies. In patients undergoing elective endovascular abdominal aortic aneurysm repair, for which there is a recognised pronounced pro-inflammatory response, a single preoperative dose of methylprednisolone (30 mg kg−1) reduced serum pro-inflammatory biomarkers (IL-6, IL-8, and CRP) and increased concentrations of the anti-inflammatory cytokine IL-10.23 The anti-inflammatory benefits of perioperative glucocorticoids have also been reported in colorectal, hepatobiliary, and orthopaedic lower-limb joint replacement surgeries. There is also evidence showing reduced pulmonary complications without affecting duration of stay or incidence of infection.24
The inflammatory–immune response is strongly activated in cardiac surgery with high peak values measured for CRP and IL-6.1 High-dose glucocorticoid administration (methylprednisolone 30 mg kg−1 or dexamethasone 1 mg kg−1) before CPB leads to a significant decrease in pro-inflammatory mediators (IL-6, IL-8, TNF, and CRP). Glucocorticoid use has been associated with a reduction in duration of postoperative mechanical ventilation, postoperative infection, hyperthermia, and length of stay after cardiac surgery.
Concerns remain regarding the possibility of a glucocorticoid-induced hyperglycaemic response, and caution should be taken, especially in diabetic patients, where no conclusive data exist. Perioperative outcomes in patients with a reduced cortisol response to surgery, such as elderly, frail, depressed, or critically ill patients, may be worse if supplemental glucocorticoids are not given.
Nutrition and fluids management
Enhanced recovery after surgery is a combination of measures that, when used together, reduce the component parts of the surgical stress response to improve postoperative outcomes and recovery times.25 There is an emphasis on a multidisciplinary approach to measures, including multimodal optimal analgesia, early mobilisation, minimally invasive surgical techniques, and early enteral feeding. Hyper-metabolism and hyper-catabolism occur after surgical tissue injury in an attempt to restore homeostasis within the body. This may lead to impaired immune functio.n, impaired wound healing, muscle wasting, and a change in nutrition requirements. Malnutrition and underfeeding are risk factors for postoperative complications.
A reduction in fasting time and preoperative carbohydrate loading have been shown to reduce the amount of fluid administered during surgery and reduce postoperative insulin resistance and associated catabolism.25 Immunonutrition with glutamine, arginine, and omega-3 fatty acid supplementation may reduce immune-mediated changes seen after surgery. However, no clear recommendation currently exists for enteral supplementation as evidence of the benefit is insufficient.25
Conclusions
The stress response to surgery is a complex neuroendocrine–metabolic and inflammatory–immune process. The physiological changes that this stress induces in the body have been discussed; it triggers a catabolic cascade with the release of growth factors and energy substrates. There is a release of inflammatory mediators, suppression of anabolic hormones, and sodium and fluid retention. The increase in sympathetic tone and its physiological sequelae plays a key role. Knowledge in this area is continuing to develop, and it is becoming clear that overexpression of inflammatory mediators and immune suppression puts the patient at increased risk of perioperative complications. A major aim of perioperative care should be to attenuate this response by the judicious selection and conduct of anaesthetic techniques.
Declaration of interests
DJB is a member of the editorial board of the British Journal of Anaesthesia.
MCQs
The associated MCQs (to support CME/CPD activity) are accessible at www.bjaed.org/cme/home for subscribers to BJA Education.
Biographies
Donal Buggy MD, MSc, DME, FRCPI, FFSEM, FCAI, FRCA is professor of anaesthesiology and perioperative medicine at University College Dublin and consultant at Mater University Hospital. He is on the editorial board of the British Journal of Anaesthesia, chairman of the European Society of Anaesthesiology (ESA) Onco-Anaesthesiology Research Group, and was a member of the ESA Research Committee from 2015 to 2019.
Barbara Cusack MCAI, PDE is a specialist registrar in anaesthesia and intensive care medicine in Ireland. She has a particular interest in clinical education and intensive care. She has completed a postgraduate diploma in clinical education at the University of Galway and an honours degree in chemistry at Trinity College Dublin.
Matrix codes: 1A01, 2C04, 3I00
References
- 1.Watt D.G., Horgan P.G., McMillan D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362–380. doi: 10.1016/j.surg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Desborough J.P. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood L. The peripheral endocrine glands. In: Sherwood L., editor. Human physiology: from cells to systems. 9th Edn. Cengage Learning; Belmont, CA: 2016. pp. 112–119. [Google Scholar]
- 4.Walker J.J., Terry J.R., Lightman S.L. Origin of ultradian pulsatility in the hypothalamic–pituitary–adrenal axis. Proc Biol Sci. 2010;277:1627–1633. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manou-Stathopoulou V., Korbonits M., Ackland G.L. Redefining the perioperative stress response: a narrative review. Br J Anaesth. 2019;123:570–583. doi: 10.1016/j.bja.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Raber J. Detrimental effects of chronic hypothalamic–pituitary–adrenal axis activation. From obesity to memory deficits. Mol Neurobiol. 1998;18:1–22. doi: 10.1007/BF02741457. [DOI] [PubMed] [Google Scholar]
- 7.Wall T., Sherwin A., Ma D., Buggy D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123:135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duff S., Connolly C., Buggy D.J. Adrenergic, inflammatory, and immune function in the setting of oncological surgery: their effects on cancer progression and the role of the anaesthetic technique in their modulation. Int Anesthesiol Clin. 2016;54:48–57. doi: 10.1097/AIA.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 9.Alazawi W., Pirmadjid N., Lahiri R., Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264:73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 10.Marik P.E., Flemmer M. The immune response to surgery and trauma: implications for treatment. J Trauma Acute Care Surg. 2012;73:801–808. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- 11.Paola A., Carlo L., Cinzia D.R. Stress response to surgery, anaesthetics role and impact on cognition. J Anesth Clin Res. 2015;6:1–5. [Google Scholar]
- 12.Iwasaki M., Edmondson M., Sakamoto A., Ma D. Anaesthesia, surgical stress, and “long-term” outcomes. Acta Anaesthesiol Taiwan. 2015;53:99–104. doi: 10.1016/j.aat.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Marana E., Colicci S., Meo F., Marana R., Proietti R. Neuroendocrine stress response in gynaecological laparoscopy: TIVA with propofol versus sevoflurane anaesthesia. J Clin Anesth. 2010;22:250–255. doi: 10.1016/j.jclinane.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Landoni G., Lomivorotov V.V., Nigro C. Volatile anaesthetics versus total intravenous anaesthesia for cardiac surgery. N Engl J Med. 2019;380:1214–1225. doi: 10.1056/NEJMoa1816476. [DOI] [PubMed] [Google Scholar]
- 15.Sulaiman S., Karthekeyan R.B., Vakamudi M., Sundar A.S., Ravullapalli H., Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 16.Wang X.W., Cao J.B., Lv B.S. Effect of perioperative dexmedetomidine on the endocrine modulators of stress response: a meta-analysis. Clin Exp Pharmacol Physiol. 2015;42:828–836. doi: 10.1111/1440-1681.12431. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hashimi M., Scott W.M., Thompson J.P., Lambert D.G. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111:80–88. doi: 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 18.Cruz F.F., Rocco P.R.M., Pelosi P. Anti-inflammatory properties of anaesthetic agents. Crit Care. 2017;21:1–7. doi: 10.1186/s13054-017-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janku F., Johnson L.K., Karp D.D. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27:2032–2038. doi: 10.1093/annonc/mdw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lattermann R., Belohlavek G., Wittmann S., Fuchtmeier B., Gruber M. The anticatabolic effect of neuraxial blockade after hip surgery. Anesth Analg. 2015;101:1202–1208. doi: 10.1213/01.ane.0000167282.65352.e7. [DOI] [PubMed] [Google Scholar]
- 21.Milosavljevic S.B., Pavlovic A.P., Trpkovic S.V. Influence of spinal and general anaesthesia on the metabolic, hormonal, and haemodynamic response in elective surgical patients. Med Sci Monit. 2014;20:1833–1840. doi: 10.12659/MSM.890981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carey B.M., Jones C.N., Fawcett W.J. Anaesthesia for minimally invasive abdominal and pelvic surgery. BJA Educ. 2019;19:254–260. doi: 10.1016/j.bjae.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de la Motte L., Kehlet H., Vogt K. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double-blind, placebo-controlled clinical trial. Ann Surg. 2014;260:540–548. doi: 10.1097/SLA.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 24.Kehlet H., Lindberg-Larsen V. High-dose glucocorticoid before hip and knee arthroplasty: to use or not to use—that’s the question. Acta Orthop. 2018;89:477–479. doi: 10.1080/17453674.2018.1475177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weimann A., Brage M., Carli F. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]