Abstract
A restricted polyclonal or biclonal gammopathy resulting in bleeding tendencies was diagnosed in a young, neutered male English bulldog with concurrent splenomegaly, anemia, and severe elevations in IgM and, to a lesser degree, IgA immunoglobulins. There was a positive clinical response to treatment with prednisone and chlorambucil. This case bears similarity to a recently published syndrome of polyclonal gammopathy that is not neoplastic in origin in this breed.
Key clinical message:
The current case describes the management and clinical course of a recently described syndrome of polyclonal gammopathy in English bulldogs.
Résumé
Gammapathie et coagulopathie progressives chez un jeune bouledogue Anglais. Une gammapathie polyclonale restreinte ou biclonale résultant en une tendance aux saignements fut diagnostiquée chez un jeune bouledogue Anglais mâle castré, avec une splénomégalie concomitante, de l’anémie et une augmentation sévère des immunoglobulines IgM et, à un degré moindre, des IgA. Une réponse clinique positive au traitement avec de la prednisone et du chlorambucil fut notée. Ce cas comporte des similarités avec un syndrome récemment décrit de gammapathie polyclonale qui ne serait pas d’origine néoplasique chez cette espèce.
Message clinique clé :
Le présent cas décrit la gestion et l’évolution clinique d’un syndrome récemment décrit de gammapathie polyclonale chez les bouledogues Anglais.
(Traduit par Dr Serge Messier)
A 2-year-old neutered male English bulldog was presented to the Tufts at Tech Community Veterinary Clinic of Tufts Cummings School of Veterinary Medicine for consultation regarding corrective surgery for entropion. The dog had a history of chronic dermatitis and keratoconjunctivitis sicca. There was also a history of mild to moderate hyperglobulinemia [ranging from 49 to 62 g/L; reference interval (RI): 25 to 45 g/L], which had been detected on 3 separate screening biochemical profiles since 1 y of age. Previous complete blood (cell) count (CBC) results for this patient had been unremarkable other than suspected thrombocytopenia with platelet clumping. There was no travel history.
Case description
On presentation (Day 1), the dog was bright, alert, and active with a mild superficial pyoderma. There was bilateral mild lower eyelid entropion with chronic corneal changes. Severe cranial organomegaly was detected on palpation. The CBC revealed a mild normocytic, normochromic anemia with a normal total leukocyte count (Table 1, Day 1). The platelet count was decreased at 49 × 103 platelets/μL (RI: 200 to 500 × 103/μL), but several small to moderately sized platelet clumps were present on the feathered edge and body of the smear. Review of the smear by a Board-certified pathologist was consistent with decreased platelet numbers. Evaluation of leukocyte morphology revealed reactive lymphocytes with a subpopulation of medium to large atypical cells with deeply basophilic cytoplasm and fine chromatin. Abnormalities on the serum biochemistry included hyperproteinemia with severe hyperglobulinemia and mild hypoalbuminemia, as well as mild total hypercalcemia (Table 1, Day 1). Dipstick and sediment analyses of urine collected by cystocentesis were unremarkable.
Table 1.
Relevant laboratory data throughout the course of disease.
| Parameter | Reference interval | Day | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 120 | 232 | 308 | 406 | ||
| Hematocrit | 40% to 55% | 37* | 30* | 38* | 32.9* (37.3 to 61.7) | 20* |
| Reticulocyte count | 0 to 100.0 × 103/μL | 10.0 | 49.5 | 78.7 | 7.9* (10 to 110) | 14.4 |
| Leukocyte count | 4.5 to 15.0 × 103/μL | 6.3 | 2.9* | 9.7 | 8.57 (5.05 to 16.76) | 7.8 |
| Lymphocyte count | 1.0 to 4.8 × 103/μL | 1.9 | 0.8* | 2.5 | 1.72 (1.05 to 5.1) | 4.4 |
| Neutrophil count | 2.6 to 11 × 103/μL | 3.8 | 1.9* | 6.4 | 6.09 (2.95 to 11.64) | 3.0 |
| Monocyte count | 0.2 to 1.0 × 103/μL | 0.2 | 0.1* | 0.5 | 0.71 (0.16 to 1.12) | 0.4 |
| Eosinophil count | 0.1 to 1.2 × 103/μL | 0.1 | 0* | 0.3 | 0.04 (0.06 to 1.23) | 0.1 |
| Platelet count | 200 to 500 × 103/μL | 49* | 126* | 175* | 44* (148 to 484) | 137* |
| Clumped platelets | Moderate | Rare | Moderate | None seen | Rare | |
| Albumin | 28 to 40 g/L | 25* | 28 | 34 | 28* | 27* |
| Globulin | 23 to 42 g/L | 84* | 85* | 52* | 68* | 70* |
| Total protein | 55 to 78 g/L | 109* | 113* | 86* | 96* | 97* |
| Total calcium | 2.3 to 2.8 mmol/L | 2.9* | 2.6 | — | 2.8 | 2.9* |
| Blood urea nitrogen | 2.5 to 9.6 mmol/L | 5.3 | 9.2 | 8.6 | 5.3 | 20.0* |
| Creatinine | 44.2 to 159.1 μmol/L | 106.1 | 79.6 | 70.7 | 256.4* | 389.0* |
| Urine specific gravity | — | 1.024 | — | — | 1.005 | 1.014 |
Day 1, at initial evaluation; Day 120, at evaluation for mucosal bleeding; Day 232, after treatment with prednisone and chlorambucil; Day 308, at time of leptospirosis diagnosis; Day 406, at the time of euthanasia. Prednisone and chlorambucil had been discontinued at the time of leptospirosis diagnosis. Complete blood (cell) counts (CBC), except for Day 308, were performed at Colorado State University Veterinary Diagnostic Laboratory. The CBC on Day 308 and all serum biochemistries were performed in-house (Procyte Dx, Catalyst Dx, IDEXX). In-house reference values for CBC results for Day 308 are listed in parentheses after each result. Urine specific gravity was determined manually. Results outside of the reference ranges are marked with an asterisk (*).
Due to concern for infectious disease, serology for Ehrlichia canis, Ehrlichia ewingii, Anaplasma phagocytophilum, Anaplasma platys, and Borrelia burgdorferi, and antigen detection of Dirofilaria immitis using the SNAP 4Dx Test were performed. These tests were all negative. Agarose gel serum protein electrophoresis (SPE) was performed by the reference laboratory (IDEXX, Westbrook, Maine, USA) and showed an elevated beta globulin fraction characterized by 2 narrow split peaks within this fraction, consistent with a biclonal gammopathy. A precipitation-based qualitative Bence-Jones protein screen (IDEXX) was negative for Bence-Jones proteinuria. The blood pressure was normal. Abdominal radiographs showed a mass effect in the region of the spleen with decreased serosal detail. Radiographs of the thorax, spine, and extremities were unremarkable. The dog was referred to the Internal Medicine Department at the Tufts Cummings Foster Hospital for Small Animals for ultrasonography and bone marrow sampling.
Bone marrow aspirate samples were taken from the humerus. Evaluation by a Board-certified clinical pathologist revealed a hypercellular marrow with increased numbers of occasionally mildly dysplastic megakaryocytes, likely associated with hyperplasia. The myelocyte:erythrocyte (M:E) ratio was 1.0; maturation of both lines was orderly and to completion. Lymphocyte and plasma cell populations were morphologically unremarkable and were less than 2% of the nucleated cell population. The bone marrow response to the peripheral cytopenias appeared appropriate.
Abdominal ultrasound revealed severe splenomegaly with a coarse echotexture and a single hypoechoic splenic nodule. The liver was diffusely hypoechoic. There was a small volume of peritoneal effusion. Ultrasound-guided fine-needle aspirates of the liver and spleen were obtained and reviewed by a Board-certified clinical pathologist. On hepatic cytology, there were rare clusters of hepatocytes, which were moderately to markedly hemodiluted and only contained low numbers of nucleated cells. The numbers of lymphocytes exceeded hepatocytes, and 75% of the lymphocytes were small-sized and 25% were medium-sized. Overall, the diagnostic quality was marginal, and definitive interpretation was not possible.
Splenic nodule and parenchymal cytology both demonstrated a predominance of monomorphic medium-sized lymphocytes (80% to 85%) with round to oval or indented nuclei with coarse to clumped chromatin (without distinct nucleoli) and small to moderate amounts of grainy, pale basophilic cytoplasm. This finding raised a concern for lymphoma, although aspiration of a large germinal center in the spleen could not be ruled out. There was a mild increase in the number of plasma cells. Plasma cells were well-differentiated, and there was no concern for a plasma cell neoplasm cytologically.
Splenic aspirates were sent to the Clinical Immunology Laboratory at Colorado State University for further testing. Flow cytometry was performed as previously described (1). Small lymphocytes accounted for 69% of cells in the sample and included a mixture of CD21+ B-cells, CD4+ T-cells and CD8+ T-cells. While a plasma cell neoplasia was a primary differential diagnosis in this case, flow cytometry could not be used to affirmatively identify plasma cells, because antibodies specific for this cell type are not available for dogs. Polymerase chain reaction (PCR) for antigen receptor rearrangements (PARR) (2) revealed a polyclonal lymphocyte population (Figure 1A). Neither test was supportive of neoplasia, so an infectious disease process was suspected. Doxycycline (Cadila Healthcare, Ahmedebad, India) at 10 mg/kg body weight (BW), PO, q12h was prescribed to empirically treat for undetected tickborne infection but was discontinued 1 wk into therapy due to gastrointestinal upset. A CBC and serum biochemistry performed after 1 wk of doxycycline therapy demonstrated no significant changes in hematocrit or globulin levels. The platelet count was decreased at 101 × 103/μL (RI: 200 to 500 × 103/μL) with moderate platelet clumping present. A PCR panel to further screen for tickborne diseases including Babesia, Anaplasma, Ehrlichia, Heptatozoon, Leishmania, Neorickettsia, Bartonella, Mycoplasma haemocanis, and Mycoplasma haematoparvum was performed, and the results were negative for all (IDEXX).
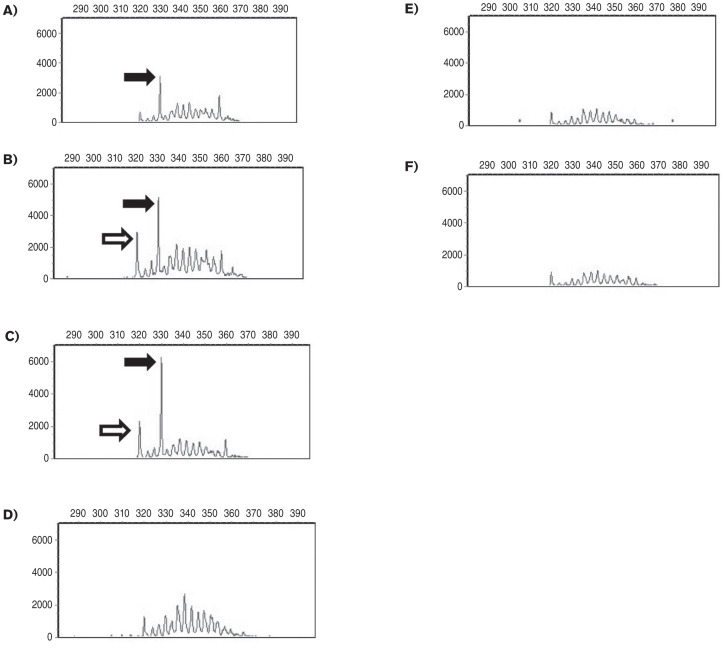
Figure 1.
Results of the sequential PCR for antigen receptor rearrangements (PARR) (Colorado State University Clinical Immunology Laboratory). Complete immunoglobulin heavy chain (IGH) gene rearrangement amplification of DNA from spleen and blood samples at different time points are presented. An immunoglobulin heavy chain peak is interpreted as clonal if the amplitude of the peak is greater than 5000 and the peak is greater than 3 times the height of the base peaks forming the polyclonal background. Amplicon size is displayed along the horizontal axis and abundance of amplicons is displayed on the vertical axis. A — Splenic aspirates during initial evaluation period, Day 71; patient was asymptomatic. A suspicious IGH rearrangement (solid arrow) among a polyclonal background was detected in the spleen, which did not reach clonal criteria. B — Peripheral blood during initial evaluation period, Day 101; patient was asymptomatic. An IGH rearrangement of identical size to that in the spleen was again detected in the blood but did not reach clonal criteria (solid arrow). A second suspicious IGH rearrangement (open arrow) was also detected in blood. C — Peripheral blood at recheck examination for mucosal bleeding, Day 120. The previously detected IGH rearrangement was again present and reached clonal criteria in this sample (solid arrow). The second suspicious IGH rearrangement (open arrow) was also detected in this sample but did not meet criteria for clonality. D — Peripheral blood at recheck examination after treatment with prednisone and chlorambucil, Day 232. The patient’s symptoms had resolved. The 2 dominant rearrangements diminished proportionally in amplitude, and no IGH rearrangements met clonal criteria. These findings persisted at the time of euthanasia, Day 406, in both the blood (E) and the spleen (F).
Because of ongoing clinical concern for neoplasia, peripheral blood was submitted for flow cytometry and PARR analysis. Flow cytometry of the blood revealed a mild expansion of small-sized CD21+ B-cells (Table 2, Day 101). In the PARR analysis on the blood (Figure 1B) no immunoglobulin heavy chain (IGH) rearrangement met the criteria for clonality, consistent with the prior PARR of the spleen (Figure 1A). In both the splenic and blood samples there were suspicious IGH peaks among a polyclonal background that did not reach the criteria for clonality (Figure 1A, B). An IGH peak is considered clonal if the peak height is greater than 5000 and greater than 3 times the height of the peaks forming the polyclonal background.
Table 2.
Sequential flow cytometry results from peripheral blood (Colorado State University Clinical Immunology Laboratory).
| Cell type | Reference interval (/μL) | Day | |||
|---|---|---|---|---|---|
|
| |||||
| 101 | 120 | 232 | 406 | ||
| CD4 T-cell subset | 306 to 2063 | 472 | 160* | 776 | 733 |
| CD8 T-cell subset | 157 to 965 | 223 | 87* | 466 | 335 |
| CD3 Pan T-cell | 594 to 3383 | 557 | 235* | 1183 | 1100 |
| CD5 Pan T-cell | 593 to 3375 | 806 | 293* | 1348 | 1209 |
| CD21 B-cell | 0 to 724 | 1420* | 626 | 708 | 2808* |
Day 101, during initial evaluation period. Patient was asymptomatic. There was a mild expansion of small B-cells, which was polyclonal by PARR. Day 120, recheck examination for spontaneous mucosal bleeding, malaise, and decreased appetite. There was a mild decrease in T-cells, which is a non-specific finding. B-cells were detected and were within the reference interval. Day 232, recheck examination after treatment with prednisone and chlorambucil. Clinical symptoms had resolved. There was a normal population of lymphocytes with no evidence of B-cell expansion. Day 406, time of euthanasia. Patient had not received prednisone or chlorambucil for the preceding approximately 100 days due to leptospiral infection. An expansion of small B-cells was present — this population was polyclonal by PARR. Values outside of the reference ranges are indicated with an asterisk (*).
Agarose gel SPE and immunofixation using anti-canine immunoglobulin class specific reagents were performed at Colorado State University as previously described (3–5). Most globulins migrated in the beta fractions, forming 2 distinct bands within a broad smear, consistent with previous SPE results. Immunofixation showed notably increased IgM heavy chain labeling which could be resolved as a pair of restricted bands with sufficient serum dilution and mild reducing conditions (Fluidil, Sebia, France), less intense polyclonal IgA heavy chain labeling, scant polyclonal IgG heavy chain labeling, lack of demonstrable IgG4 heavy chain and a concurrent light chain presence for all heavy chains (Figure 2A).
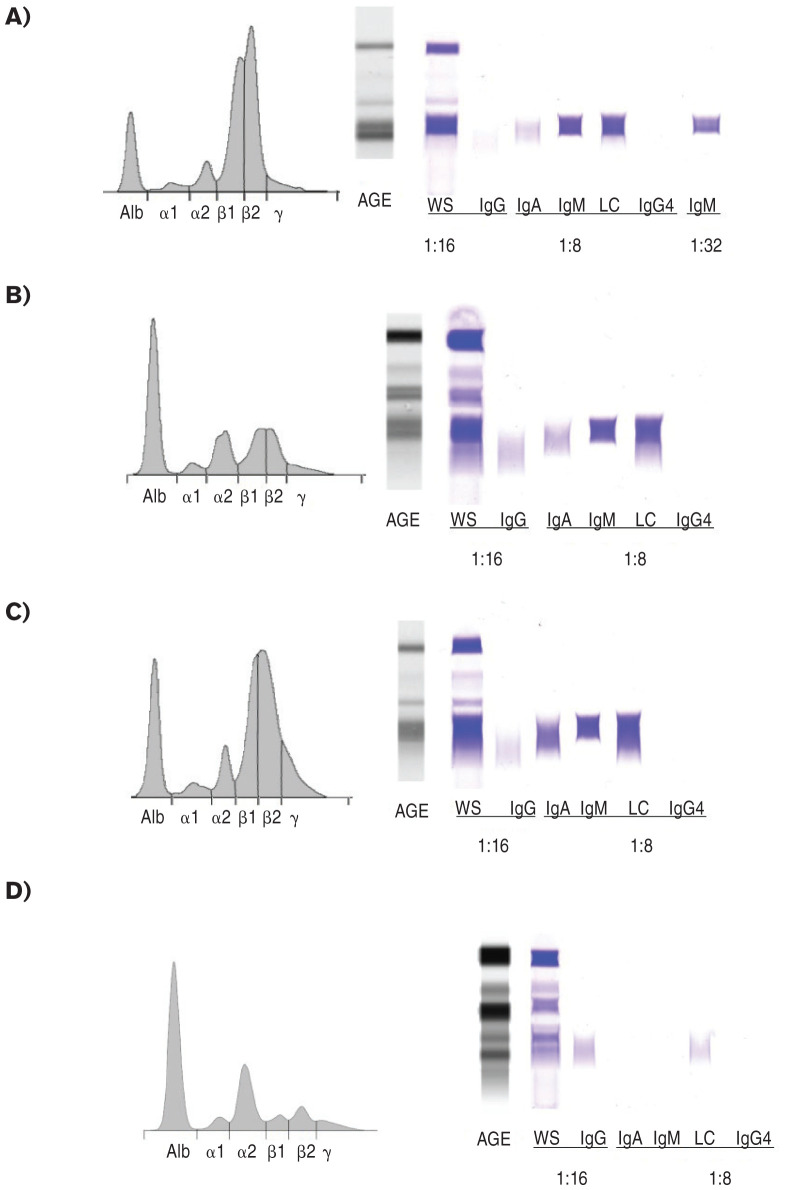
Figure 2.
Agarose gel serum protein electrophoresis and immunofixation electrophoresis of whole serum. For the English bulldog at each time point (A–C) and a normal control dog (D), the electrophoretic tracing (left) and images of the AGE gel (center) and immunofixation gel (right) are provided. A — During the initial asymptomatic period, Day 101, a pair of β1 and β2 restricted bands was present (Beta 1 fraction 41.6 g/L; RI: 3.7 to 8.1 g/dL; Beta 2 fraction 44.2 g/L; RI: 3.9 to 8.1 g/dL). Densitometric quantification of the pair of restricted bands accounted for 70.8 g/L of protein and may be an over-estimation because of the polyclonal background. Immunofixation demonstrated decreased IgG heavy chain labeling, absent IgG4 heavy chain, increased IgA heavy chain labeling and prominent IgM heavy chain labeling, which required additional serum dilution to resolve as a pair of restricted bands that correlate with the β1 and β2 restrictions. B — During treatment with prednisone and chlorambucil, Day 232, β1 and β2 restricted bands persisted, but at much lower concentrations (Beta 1 fraction 15.7 g/L, RI: 3.7 to 8.1 g/L; Beta 2 fraction 11.6 g/L, RI: 3.9 to 8.1 g/L; densitometric quantitation of both bands 20.7 g/L). Increased polyclonal IgA labeling was detected. C — At the time of euthanasia, Day 406, globulins were increased and restricted β1 and β2 IgM proteins appear to have increased (Beta 1 fraction 23.3 g/L; RI: 3.7 to 8.1 g/L,; Beta 2 fraction 37.0 g/L; RI: 3.9 to 8.1 g/L; densitometric quantitation of both bands 48.4 g/L). However, there were increased amounts of polyclonal IgA labeling and increased proteins in the gammaglobulin region (12.2 g/L, RI: 3.3 to 7.6 g/L), giving the overall SPE tracing a more polyclonal appearance as compared to diagnosis. D — The electrophoretogram and images of the AGE gel and immunofixation study from a healthy 12-year-old female spayed golden retriever dog are provided to demonstrate normal findings. Alb — albumin; AGE — amido black stained agarose electrophoresis gel; WS — anti-whole serum; IgG — anti-canine IgG heavy chain; IgA — anti-canine IgA heavy chain; IgM — anticanine IgM heavy chain; LC — anti-canine light chain; IgG4 — anticanine IgG4 heavy chain.
Evaluating the SPE in light of immunofixation findings indicated a pair of IgM restrictions, consistent with an IgM biclonal gammopathy or atypical restricted polyclonal gammopathy. Quantification of IgA, IgM, and IgG proteins was performed using commercial canine-specific enzyme-linked immunosorbent assays (ELISA) (3). The results showed marked IgM gammopathy (> 100 g/L; control dogs, range: 1.3 to 4.6 g/L), IgA gammopathy (50.5 g/L; control dogs, range: 0.6 to 9.3 g/L), and IgG concentrations slightly below the reference interval (7.48 g/L; control dogs, range: 10.3 to 32.8 g/L). Based on lack of clonality by PARR and the increase in 2 different immunoglobulin isotypes by immunofixation and ELISA, neoplastic lymphoproliferative disease was considered unlikely in this patient. The dog continued to be asymptomatic, so the owners elected to monitor for progressive disease without further therapy.
The dog was presented again on Day 120 for gingival bleeding, hematemesis, and hematochezia, which had been observed intermittently for 1 wk. There was mild hyporexia and lethargy. On examination the dog was bright, alert, and responsive, and physical examination was unchanged from previous reports. A CBC showed progressive anemia, mildly decreased platelet count with rare platelet clumping, and panleukopenia. A serum biochemistry showed severe hyperglobulinemia and a mild increase in serum alanine aminotransferase (142 U/L; RI: 10 to 125 U/L) (Table 1, Day 120). Flow cytometry was repeated on a blood sample, and the CD21+ B-cell count was within the reference interval (Table 2, Day 120). Repeat PARR of peripheral blood indicated a clonally rearranged immunoglobulin gene in a polyclonal background, raising the possibility of an emerging B-cell or plasma cell neoplasm (Figure 1C). This immunoglobulin gene rearrangement had been detected on prior samples but had not previously reached the threshold for clonality (Figure 1A, B).
Treatment with prednisone (Jubilant Cadista Pharmaceuticals, Salisbury, Maryland, USA), 1 mg/kg BW, PO, q12h, and omeprazole (1 mg/kg BW, PO, q12h) was initiated and resulted in resolution of the mucosal bleeding. Two weeks later, the dog had lost 4 kg of body weight and had diffuse muscle wasting, and the owners described marked polyuria and polydipsia associated with the initiation of corticosteroid treatment. The prednisone dose was decreased to 1 mg/kg BW q24h, and chlorambucil (Wedgewood Pharmacy, Swedesboro, New Jersey, USA) was added at 5 mg/m2, PO, q48h. The owners reported that the dog’s polyuria and polydipsia resolved with the decrease in the prednisone dosage.
Serum biochemistry, CBC, and clinical condition were monitored monthly. No further bleeding was observed, and the dog’s energy level and appetite returned to normal levels. After 112 d of treatment with oral prednisone and chlorambucil, muscle wasting and palpable splenomegaly had resolved. There was a persistent mild nonregenerative anemia, mild thrombocytopenia with moderate platelet clumping, and the neutropenia resolved. Hyperglobulinemia persisted but was mild (Table 1, Day 232). Repeat flow cytometry on blood demonstrated a normal mixed population of predominantly small lymphocytes with no evidence of B-cell expansion (Table 2, Day 232). The PARR did not show any clonally rearranged immunoglobulin gene (Figure 1D). The SPE and immunofixation found persistence of the pair of IgM restricted bands with increased IgA labeling but at a much lower concentration (Figure 2B). Prednisone was tapered to 0.5 mg/kg BW, PO, q24h and chlorambucil was continued at the initial dosage.
The dog was returned on Day 308 because of progressive polyuria, polydipsia, and lethargy over the previous 2 wk. The owners had discontinued prednisone at the onset of symptoms due to concern that the clinical signs were related to this treatment. Another dog in the household had been euthanized 2 wk earlier for acute kidney injury of unknown etiology. Both dogs were current on vaccination against rabies, distemper virus, adenovirus, parainfluenza virus, and parvovirus but had not previously been vaccinated against leptospirosis due to their urban lifestyle and limited access to the outdoors. A CBC showed non-regenerative anemia and thrombocytopenia with no platelet clumps observed. On serum biochemistry there was a mild creatinine elevation, normal blood urea nitrogen, mild hypokalemia (3.4 mmol/L; RI: 3.5 to 5.8 mmol/L), mild hypochloremia (107 mmol/L; RI: 109 to 122), and moderate hyperglobulinemia (Table 1, Day 308). Urine was hyposthenuric with proteinuria (5 g/L). Hematuria, pyuria, and numerous rod-shaped bacteria were present on sediment examination. Aerobic urine culture was negative for growth. Leptospirosis antibody panel by microagglutination was strongly positive for Leptospira grippotyphosa at a dilution of 1:51 200 (IDEXX). Treatment was initiated with amoxicillin/clavulanic acid (Dechra Pharmaceuticals, Norwich, UK), 13.6 mg/kg BW, PO, q12h for 7 d followed by doxycycline at 5 mg/kg BW, PO, q12h for 14 d in addition to supportive care with antiemetics and isotonic fluids. Chlorambucil was discontinued due to concern for immune suppression.
The owners elected euthanasia 98 d after diagnosis of leptospirosis (Day 406) due to progressive azotemia (Table 1, Day 406) and worsening quality of life. No immunosuppressive medications (chlorambucil or prednisone) had been administered since the diagnosis of leptospirosis. Samples of blood, bone marrow, and spleen were collected at the time of euthanasia. The SPE and immunofixation demonstrated that, without treatment, the IgM restricted bands returned, and there were also increased amounts of polyclonal IgA labeling and an increased gamma globulin fraction with modestly increased IgG and IgG4 labeling (Figure 2C). Flow cytometry of splenic tissue revealed a heterogenous population of small-sized B-cells and T-cells, in similar proportions to the first splenic sample. Flow cytometry on the bone marrow revealed small proportions of heterogenous small-sized phenotypically normal B-cells and T-cells. On peripheral blood, a homogenous expansion of small B-cells was reported (Table 2, Day 406). There was no B-cell clonality detected by PARR on any sample (Figure 1E, F). In the bone marrow and peripheral blood, T-cell receptor clonality was detected. The significance of this finding is not clear given lack of evidence of a neoplastic T-cell population on flow cytometry in any sample.
On necropsy examination, the spleen was diffusely enlarged by multifocal coalescing, markedly enlarged, lymphoid nodules (Figure 3A). Most of the lymphocytes were small to intermediate in size with fewer admixed large lymphocytes (Figure 3B). By immunohistochemistry, the lymphoid nodules were composed of Pax5-immunoreactive B-cells, and low numbers of CD3-immunoreactive T-cells were scattered in between follicles (6). Increased numbers of MUM 1-immunoreactive plasma cells infiltrated splenic sinusoids, and there was moderate splenic congestion. In the kidneys, there was marked bilateral, diffuse, chronic lymphoplasmacytic interstitial nephritis with tubular degeneration, necrosis, and marked, multifocal interstitial fibrosis was present, consistent with chronic Leptospira infection. There was no overt evidence of glomerulonephritis. There was also mild, chronic, lymphoplasmacytic hepatitis, likely secondary to leptospirosis. The bone marrow showed adequate cellularity with cells of all lineages showing progressive maturation and no evidence of neoplasia.
Figure 3.

Histopathology of the spleen on post-mortem evaluation. A — There was marked lymphoid hyperplasia with multifocal to coalescing lymphoid nodules; bar = 200 μm. B — The lymphoid population was predominantly small to intermediate lymphocytes with fewer admixed large lymphocytes; bar = 20 μm.
Discussion
This report describes a case of severe hyperglobulinemia secondary to an expanded polyclonal B-cell population that eventually led to bleeding tendencies in a young English bulldog. Progressive hyperglobulinemia had been detected incidentally in this patient as early as 1 y of age. When serum protein electrophoresis (SPE) was first performed at 2 y of age, it demonstrated a pair of restricted bands in the beta fractions suggesting a biclonal gammopathy and neoplasia. Immunofixation was useful in further characterizing this gammopathy. Biclonal gammopathies are typically associated with the presence of either different dimerization/multimerization states of a single monoclonal paraprotein or 2 different immunoglobulin clones. The electrophoretic profile and immunofixation patterning, however, were atypical for a neoplastic process, as there was not concurrent suppression of the non-involved immunoglobulin fractions in the face of high concentration IgM restriction (7,8).
It is possible that chronic infection could create polyclonal background “noise” in this patient to make a diagnosis of a monoclonal gammopathy difficult, as suggested in another case report (9). At the initial examination, however, this patient had no known history of infectious disease and was negative for the common regional tickborne infections. Chronic skin disease was present but was mild. Chronic leptospirosis could be considered as a cause for antigenic stimulation, but the chronicity and pattern of immunoglobulin involvement were atypical for leptospirosis. Leptospira infection begins with an IgM response which quickly progresses to IgG (10). In this case, the post-treatment gammopathy was characterized by a return of IgM and IgA without significant involvement of the IgG typically produced with an anamnestic response. Polyclonal immunoglobulin expansion with a distinctly restricted electrophoretic band which mimics a monoclonal gammopathy by SPE is associated with several non-neoplastic conditions in human and veterinary cases (4,11–13). The electrophoretic and immunofixation pattern in this case could suggest either a non-neoplastic restricted polyclonal gammopathy or an atypical presentation of a biclonal gammopathy.
Other factors in this case were not supportive of a neoplastic process. The dog had developed hyperglobulinemia at an early age (< 1 y of age), whereas monoclonal gammopathy due to neoplastic disease in dogs is typically diagnosed later in life (14,15). Flow cytometry and PARR were used to evaluate this patient for a B-cell expansion and/or a clonal B-cell/ plasma cell population, respectively. In most samples, the flow cytometry results demonstrated a normal and heterogenous population of lymphocytes. There was, however, a monomorphic expansion of small-sized B-cells at 2 time points: at the time of initial diagnosis and at the time of euthanasia (Table 2, Days 101 and 406). However, corresponding PARR results for these samples demonstrated that these lymphocytes were polyclonal (Figure 1B, F). This B-cell population did reach the threshold criteria for clonality by PARR at the time of presentation for bleeding diathesis (Figure 1C), but a neoplastic B-cell expansion at this time point was not supported by flow cytometry (Table 2, Day 120). At the time of euthanasia, PARR results demonstrated a clonal T-cell rearrangement on samples of blood and bone marrow, but this is again difficult to interpret given the lack of a neoplastic T-lymphocyte population on flow cytometry. Histopathology of the spleen, liver, mesenteric lymph nodes, and bone marrow at the time of euthanasia also were not consistent with neoplasia in this patient.
The patient in this report was presented with clinical symptoms of coagulopathy, demonstrated by mucosal bleeding, which may be attributed to severe hyperglobulinemia. Bleeding tendencies have been reported in canine cases of monoclonal gammopathy, most frequently with Waldenström’s macroglobulinemia and multiple myeloma (7,16); however, to the authors’ knowledge, there is no report of bleeding diathesis related to a polyclonal gammopathy in the dog. The mechanism of bleeding tendencies in hyperglobulinemic patients is complex and likely multifactorial. Myelophthisis could lead to thrombocytopenia in cases of neoplastic disease, but the bone marrow aspirate in this patient was not suggestive of this. Additionally, this dog’s platelet concentrations were not likely to be low enough to account for spontaneous bleeding. Thrombocytopathia may occur through paraprotein coating and inhibition of platelet aggregation; acquired von Willebrand’s Disease due to macrophage-mediated clearance of immune complexes of von Willebrand’s factor with autoantibodies may also be a factor. Other mechanisms for coagulopathy include prevention of fibrin polymerization due to binding of paraproteins to fibrin monomers and excessive fibrinolysis (17,18).
Hyperviscosity syndrome may also be seen in patients with severe hyperglobulinemia, particularly when there is a significant elevation in IgM, as in this case, due to the molecules’ tendency to travel through the bloodstream in pentamers (7). Hyperviscosity syndrome commonly results in bleeding tendencies due to the high shear force of blood and subsequent rupture of small capillaries, particularly in the nose, mouth, eyes, and skin (19). Although most commonly reported in cases of monoclonal IgM gammopathy, hyperviscosity syndrome has been well-documented in human polyclonal gammopathies (20).
If this dog had an atypical presentation of a true biclonal gammopathy, Waldenström’s macroglobulinemia is a primary differential diagnosis. This is an uncommon disorder characterized by a neoplastic population of lymphoplasmacytic cells in the bone marrow and a monoclonal IgM gammopathy. Bleeding tendencies are more common with this disease than in other types of monoclonal gammopathies, and up to 10% to 30% of human patients have associated hyperviscosity syndrome (19). Bleeding tendencies have also been reported in canine cases of Waldenström’s macroglobulinemia (7,16). However, on bone marrow aspirates from this dog, the number and morphology of plasma cells were within normal limits, and there was no cytological evidence of a plasma cell neoplasm. Bone marrow core biopsy prior to treatment may have been helpful to more definitively exclude this differential diagnosis before initiating treatment but was not pursued due to clinical bleeding tendencies and owner financial limitations. The absence of lytic bone lesions or a neoplastic plasma cell population in liver, spleen, or lymph node samples further argues against a plasma cell neoplasm in this dog.
There was a positive response to treatment of the gammopathy with a metronomic dosing schedule of chlorambucil in combination with prednisone. Chlorambucil is an oral alkylating antineoplastic and immunosuppressive agent that has shown efficacy in the treatment of various types of chronic lymphoproliferative diseases in both canine and human patients, often in combination with prednisone (21–23). Metronomic low-dose daily or every 48-hour dosing protocols have been described in dogs with reasonable efficacy against various types of cancer while causing minimal adverse events (24,25).
Recently, an unusual B-cell lymphoproliferative disease of English bulldogs was described, which is characterized by a polyclonal B-cell expansion with concurrent hyperglobulinemia, anemia, and splenomegaly. Most of these cases are asymptomatic with an indolent clinical course. Age at diagnosis varies, with a median age of 7.3 y (range 2.0 to 13.5 y), and males are over-represented. It is hypothesized to be genetic and not neoplastic in origin; however, the etiology is unknown (26). Polyclonal gammopathies of genetic etiology such as persistent polyclonal B-cell lymphocytosis and inherited hyper-IgM syndrome are reported in the human medical literature (27,28), but data in the canine population are largely lacking. Both of these human conditions demonstrate polyclonal gammopathies that are the result of genetic mutation leading to lymphocytic expansion without an underlying infectious or neoplastic disease.
In conclusion, the current case demonstrates a dog with an atypical electrophoretic profile that was initially polyclonal by PARR and clinically asymptomatic for an extended period of time. When left untreated, this dog eventually developed a bleeding diathesis associated with high serum levels of IgM, and the possibility of emerging neoplastic lymphoproliferative disease was suggested based upon PARR results. There was a positive clinical response to treatment with chlorambucil and prednisone until the patient contracted leptospirosis. At the time of euthanasia, there was no clear evidence of B-cell or plasma cell neoplasia despite discontinuation of treatment approximately 100 d earlier and return of the gammopathy. This case bears close similarity to a recently published syndrome of polyclonal gammopathy in English bulldogs that is not well understood (26). As we continue to learn about this disease process, close monitoring and immunologic testing at regular intervals may be warranted to detect the emergence of a neoplastic process. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Rout ED, Avery PR. Lymphoid neoplasia correlations between morphology and flow cytometry. Vet Clin North Am Small Anim Pract. 2017;47:53–70. doi: 10.1016/j.cvsm.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Waugh EM, Gallagher A, Haining H, et al. Optimisation and validation of a PCR for antigen receptor rearrangement (PARR) assay to detect clonality in canine lymphoid malignancies. Vet Immunol Immunopathol. 2016;182:115–124. doi: 10.1016/j.vetimm.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris AD, Rout E, Avery A, Bolte D, Belling-Kelly E, Moore AR. Validation and method comparison of the use of densitometry to quantify monoclonal proteins in canine sera. Vet Clin Pathol. 2019;48:78–87. doi: 10.1111/vcp.12766. [DOI] [PubMed] [Google Scholar]
- 4.Colopy LJ, Shiu K, Snyder LA, Avery AC, Rout ED, Moore AR. Immunoglobulin G4-related disease in a dog. J Vet Intern Med. 2019;33:2732–2738. doi: 10.1111/jvim.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaghy D, Moore AR. Identification of canine IgG and its subclasses, IgG1, IgG2, IgG3 and IgG4, by immunofixation and commercially available antisera. Vet Immunol Immunopathol. 2020;221:1–7. doi: 10.1016/j.vetimm.2020.110014. [DOI] [PubMed] [Google Scholar]
- 6.Harris LJ, Rout ED, Hughes KL, et al. Clinicopathologic features of lingual canine T-zone lymphoma. Vet Comp Oncol. 2018;16:131–139. doi: 10.1111/vco.12322. [DOI] [PubMed] [Google Scholar]
- 7.Jaillardon L, Fournel-Fleury C. Waldenstrom’s macroglobulinemia in a dog with a bleeding diathesis. Vet Clin Pathol. 2011;40:351–355. doi: 10.1111/j.1939-165X.2011.00341.x. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg CB, Boria PA, Raskin RE, Lucroy MD. Effect of chemotherapy schedule on response in Waldenstrom’s macroglobulinemia in a dog. J Vet Intern Med. 2008;22:223–226. doi: 10.1111/j.1939-1676.2007.0020.x. [DOI] [PubMed] [Google Scholar]
- 9.Geigy C, Riond B, Bley CR, Grest P, Kircher P, Lutz H. Multiple myeloma in a dog with multiple concurrent infectious diseases and persistent polyclonal gammopathy. Vet Clin Pathol. 2013;42:47–54. doi: 10.1111/vcp.12018. [DOI] [PubMed] [Google Scholar]
- 10.Lizer J, Velineni S, Weber A, Krecic M, Meeus P. Evaluation of 3 serological tests for early detection of Leptospira-specific antibodies in experimentally infected dogs. J Vet Intern Med. 2018;32:201–207. doi: 10.1111/jvim.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore AR, Avery PR. Protein characterization using electrophoresis and immunofixation; a case-based review of dogs and cats. Vet Clin Pathol. 2019;48:29–44. doi: 10.1111/vcp.12760. [DOI] [PubMed] [Google Scholar]
- 12.Singh G. Oligoclonal pattern/abnormal protein bands in post-treatment plasma cell myeloma patients: Implications for protein electrophoresis and serum free light chain assay results. J Clin Med Res. 2017;9:671–679. doi: 10.14740/jocmr3049w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawad MD, Go RS, Witzig TE, Mikhael JR, Ravindran A, Murrray DL. Pseudo-monoclonal gammopathy: A report of four cases. Haematologica. 2017;102:e466–e469. doi: 10.3324/haematol.2017.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vail DM. Hematopoietic tumors. In: Ettinger SF, Feldman EC, Côté E, editors. Textbook of Veterinary Internal Medicine. 8th ed. St. Louis, Missouri: Elsevier; 2017. pp. 2065–2078. [Google Scholar]
- 15.Bromberek JL, Rout ED, Agnew MR, Yoshimoto J, Morley PS, Avery AC. Breed distribution and clinical characteristics of B cell chronic lymphocytic leukemia in dogs. J Vet Intern Med. 2016;30:215–222. doi: 10.1111/jvim.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giraudel JM, Pagés J, Guelfi J. Monoclonal gammopathies in the dog: A retrospective study of 18 cases (1986–1999) and literature review. J Am Anim Hosp Assoc. 2002;38:135–147. doi: 10.5326/0380135. [DOI] [PubMed] [Google Scholar]
- 17.Zangari M, Elice F, Fink L, Tricot G. Hemostatic dysfunction in paraproteinemias and amyloidosis. Semin Thromb Hemost. 2007;33:339–349. doi: 10.1055/s-2007-976169. [DOI] [PubMed] [Google Scholar]
- 18.Eby C, Blinder M. Hemostatic complications associated with paraproteinemias. Curr Hematol Rep. 2003;2:388–394. [PubMed] [Google Scholar]
- 19.Decaux O, Laurat E, Perlat A, Cazalets C, Jego P, Grosbois B. Systemic manifestations of monoclonal gammopathy. Eur J Intern Med. 2009;20:457–461. doi: 10.1016/j.ejim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Chen LY, Wong PC, Noda S, Collins DR, Sreenivasan GM, Coupland RC. Polyclonal hyperviscosity syndrome in IgG4-related disease and associated conditions. J Clin Case Rep. 2015;3:217–226. doi: 10.1002/ccr3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Workman HC, Vernau W. Chronic lymphocytic leukemia in dogs and cats: The veterinary perspective. Vet Clin North Am Small Anim Pract. 2003;33:1379–1399. doi: 10.1016/s0195-5616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 22.MacEwen EG, Hurvitz AI. Diagnosis and management of monoclonal gammopathies. Vet Clin North Am. 1977;7:119–132. doi: 10.1016/s0091-0279(77)50010-x. [DOI] [PubMed] [Google Scholar]
- 23.Giudice ID, Pileri SA, Rossi M. Histopathological and molecular features of persistent polyclonal B-cell lymphocytosis (PPBL) with progressive splenomegaly. Br J Haematol. 2009;144:726–731. doi: 10.1111/j.1365-2141.2008.07551.x. [DOI] [PubMed] [Google Scholar]
- 24.Leach TN, Childress MO, Greene SN, et al. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Vet Comp Oncol. 2011;10:102–112. doi: 10.1111/j.1476-5829.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 25.Taylor F, Gear R, Hoather T, Dobson J. Chlorambucil and prednisolone chemotherapy for dogs with inoperable mast cell tumors: 21 cases. J Small Anim Pract. 2009;50:284–289. doi: 10.1111/j.1748-5827.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Rout ED, Moore AR, Burnett RC, et al. Polyclonal B-cell lymphocytosis in English bulldogs. J Vet Intern Med. 2020;34:2622–2635. doi: 10.1111/jvim.15913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornet E, Lesesve JF, Mossafa G, et al. Long-term follow-up of 111 patients with persistent polyclonal B-cell lymphocytosis with binucleated lymphocytes. Leukemia. 2009;23:419–422. doi: 10.1038/leu.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leven EA, Maffucci P, Ochs HD, et al. Hyper IgM syndrome: A report from the USIDNET Registry. J Clin Immunol. 2016;36:490–501. doi: 10.1007/s10875-016-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]