Figure 2.
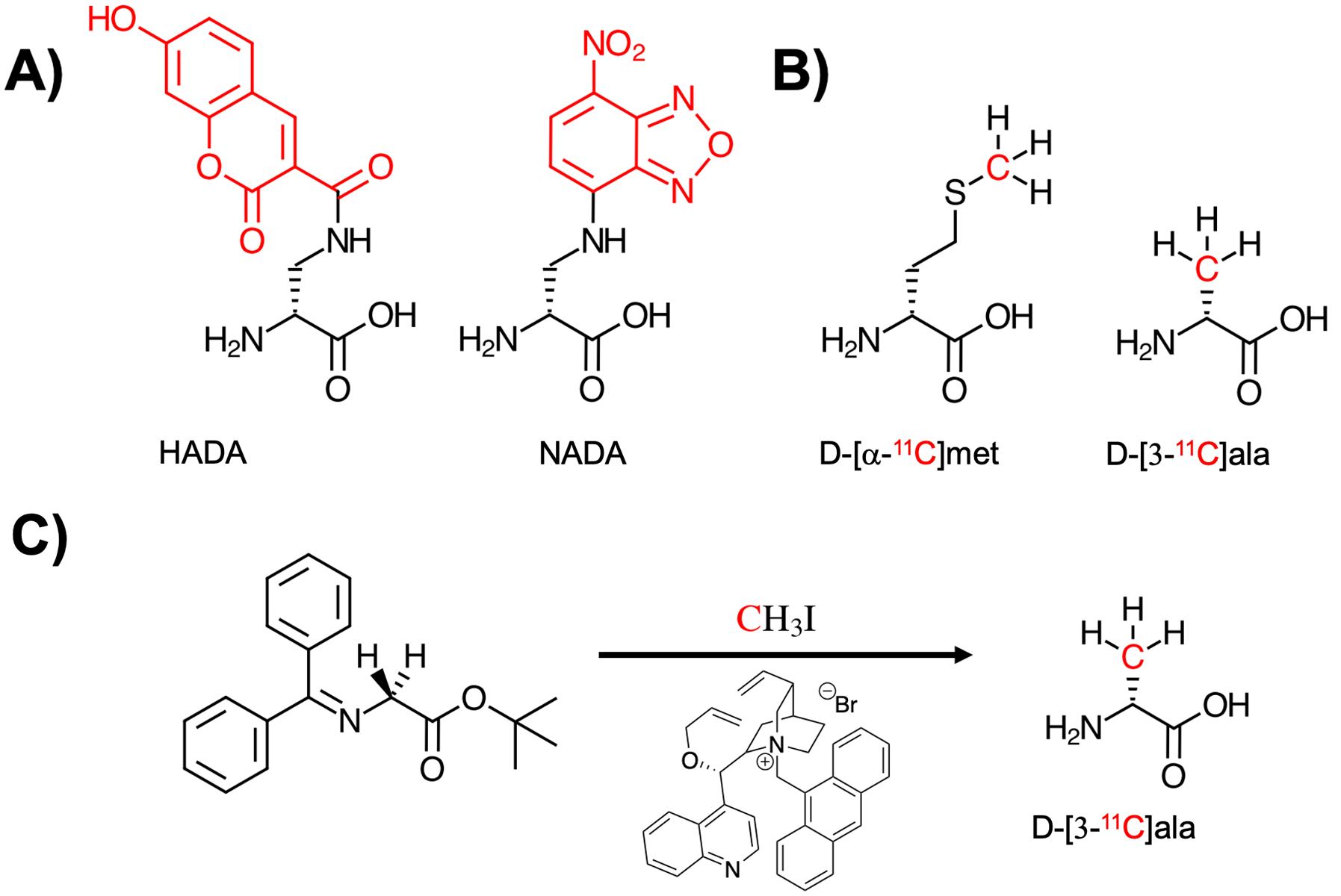
Comparison of D-amino acid derived structures modified for fluorescent detection and positron emission tomography (PET) imaging, with the sites of modification marked in red. (A) Two D-amino acid structures incorporating hydroxycoumarin and nitrobenzofuran-derived fluorophores, HADA and NADA respectively (Kuru et al. 2012). (B) Two 11C-labelled amino acids D-[α−11C]met (Neumann et al. 2017) and D-[3-11C]ala (Parker et al. 2020) with the 11C nucleus highlighted in red. (C) The enantioselective radiosynthesis of D-[3-11C]ala from an achiral glycine-derived precursor via reaction with 11C methyl iodide in the presence of a cinchonidinium-derived phase-transfer catalyst.
