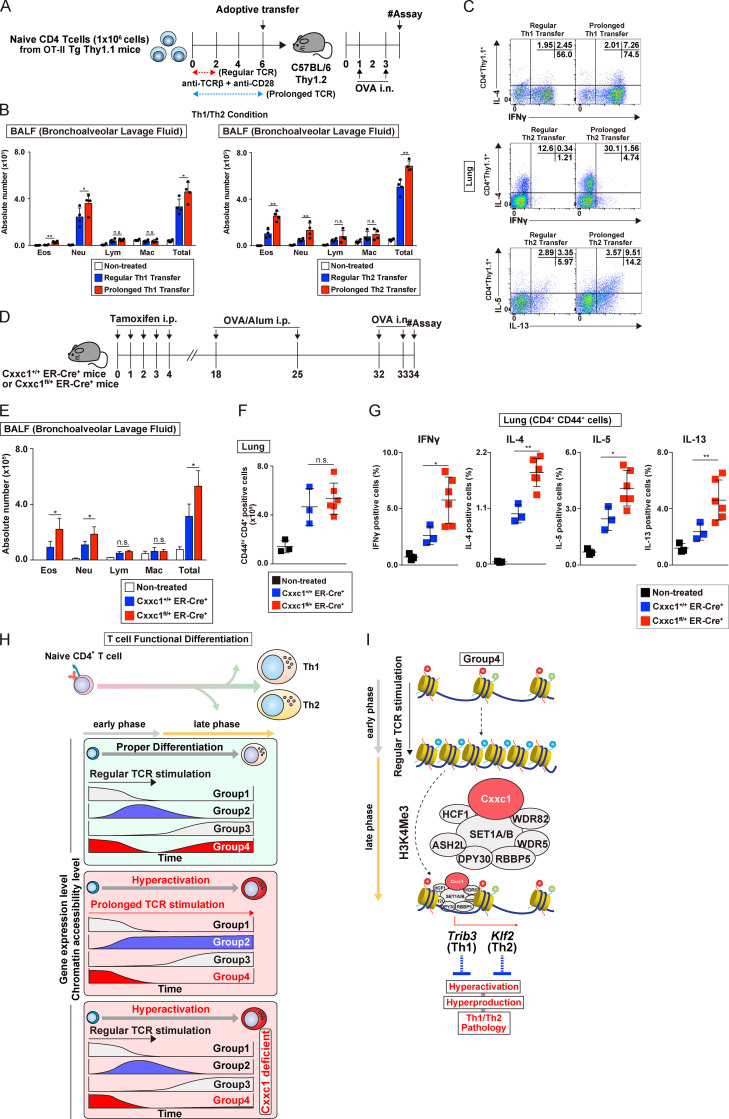
Figure 6.
Prolonged TCR/coreceptor stimulation enhances airway inflammation in vivo. (A) A schematic protocol of the allergic airway. In brief, the same numbers of Th1 or Th2 cells with or without prolonged TCR/coreceptor stimulation were transferred into C57BL/6 recipient mice, which were treated with OVA (by i.n. injection). Mice that did not undergo cell transfer were used as controls. (B) The cell numbers of eosinophils (Eos), neutrophils (Neu), lymphocytes (Lym), and macrophages (Mac) in the BAL fluid are shown as the mean values with SDs (nontreated, n = 4; Th WT, n = 4; Th with prolonged TCR/coreceptor stimulation, n = 4) are shown (*, P < 0.05; **, P < 0.01). (C) Intracellular staining profiles of IFNγ, IL-4, IL-5, and IL-13 in transferred CD4+ T cells (CD4+ Thy1.1+) in the lung. (D) An experimental protocol of allergic airway inflammation in which Cxxc1+/+ ER-Cre+ (WT) and Cxxc1fl/+ ER-Cre+ mice were treated with tamoxifen in vivo and immunized with OVA/Alum (i.p. injection). At 1 wk after immunization, these mice were challenged with OVA (i.n. administration) and analyzed. Mice without immunization were used as controls. (E) The cell numbers of eosinophils, neutrophils, lymphocytes, and macrophages in the BAL fluid are shown as the mean values with SDs (nontreated, n = 3; Cxxc1+/+ ER-Cre+, n = 3; Cxxc1fl/+ ER-Cre+, n = 6; *, P < 0.05). (F) The numbers of activated CD4+ T cells in the lung were detected by cell surface staining of CD4 and CD44. (G) The indicated cytokine-producing cells among activated CD4+ T cells (CD4+CD44+) in the lung. (nontreated, n = 3; Cxxc1+/+ ER-Cre+, n = 3; Cxxc1fl/+ ER-Cre+, n = 6; *, P < 0.05; **, P < 0.01). (H) A schematic representation of the Cxxc1-dependent epigenetic regulation of Th subset differentiation. Both onset and cessation of the TCR/coreceptor stimulation are important for gene classification (top). Expression of group 1 genes decreases upon receiving TCR/coreceptor stimulation (i.e., early phase) and never increases after cessation of the TCR/coreceptor stimulation (i.e., late phase). Group 2 genes are turned on during early phase and turned off during late phase. Group 3 genes are silenced during early phase but up-regulated during late phase. Group 4 genes, which we focused on in the present study, exhibit decreased and increased expression before and after cessation of the TCR/coreceptor stimulation, respectively. Prolonged TCR/coreceptor stimulation specifically affects expression of the groups 2 and 4 genes (middle). Without cessation of the TCR/coreceptor stimulation, group 2 and group 4 genes are constitutively active and silenced, respectively. Group 4 genes are also dysregulated in the absence of Cxxc1, resulting in decreased expression during late phase (bottom). (I) Among the group 4 genes, the chromatin structure was closed after TCR/coreceptor stimulation in the early phase and reopened after the cessation of TCR/coreceptor stimulation (later phase). Cxxc1-containing Compass complex controls the reopening of the group 4 genes, probably via its H3K4me3 function. Cxxc1-containing Compass complex binds to the Trib3 gene in developing Th1 cells and inhibits hyperproduction of IFNγ. In developing Th2 cells, Cxxc1 binds to the Klf2 gene and inhibits the hyperproduction of Th2 cytokines. n.s., not significant.