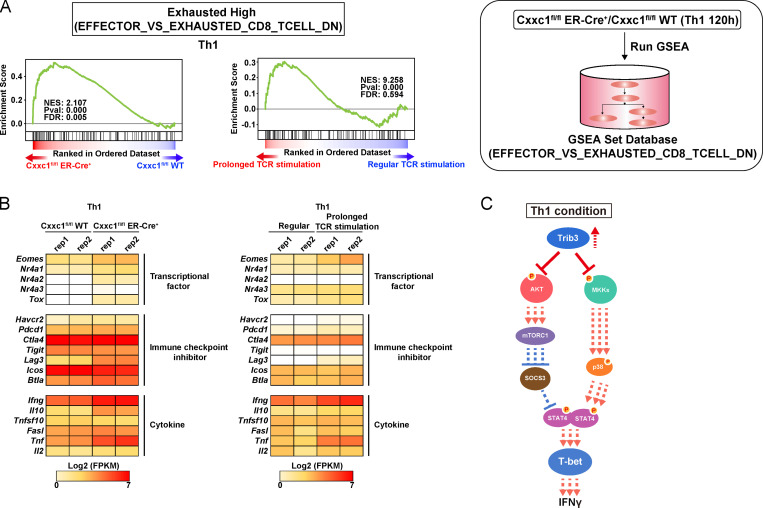
Figure S3.
Loss of Cxxc1 results in the enhanced expression of Th1 cytokines in CD4+ T cells (related to Fig. 3). (A) GSEA was performed with the gene sets down-regulated (DN) in comparison of effector CD8+ T cells with exhausted CD8+ T cells in GSEA Molecular Signatures Database. The genes were rank ordered on the basis of relative expression in Cxxc1-deficient (red) versus WT (blue) Th1 cells (left) or prolonged (red) versus regular TCR/coreceptor stimulation (blue; right). The normalized enrichment score with P value and FDR are also shown. (B) The heatmap shows the RNA expression of the genes that are reported to be dysregulated in exhausted CD8+ T cells. Genes encoding transcription factors, inhibitory receptors, and effector molecules were analyzed in WT versus Cxxc1-deficient (left) or in regular versus prolonged TCR/coreceptor stimulation (right). (C) A schematic representation of downstream signaling controlled by Cxxc1 in Th1 cells. Cxxc1 controls the IFNγ expression via Trib3-dependent phosphorylation of the Akt-mTORC1-T-bet axis and mitogen-activated protein kinase kinase (MKK)–p38–T-bet axis. Cxxc1 deletion results in hyperproduction of IFNγ due to a lack of suppression of Akt and MKKs. FPKM, fragments per kilobase of exon per million reads mapped.