Figure 4.
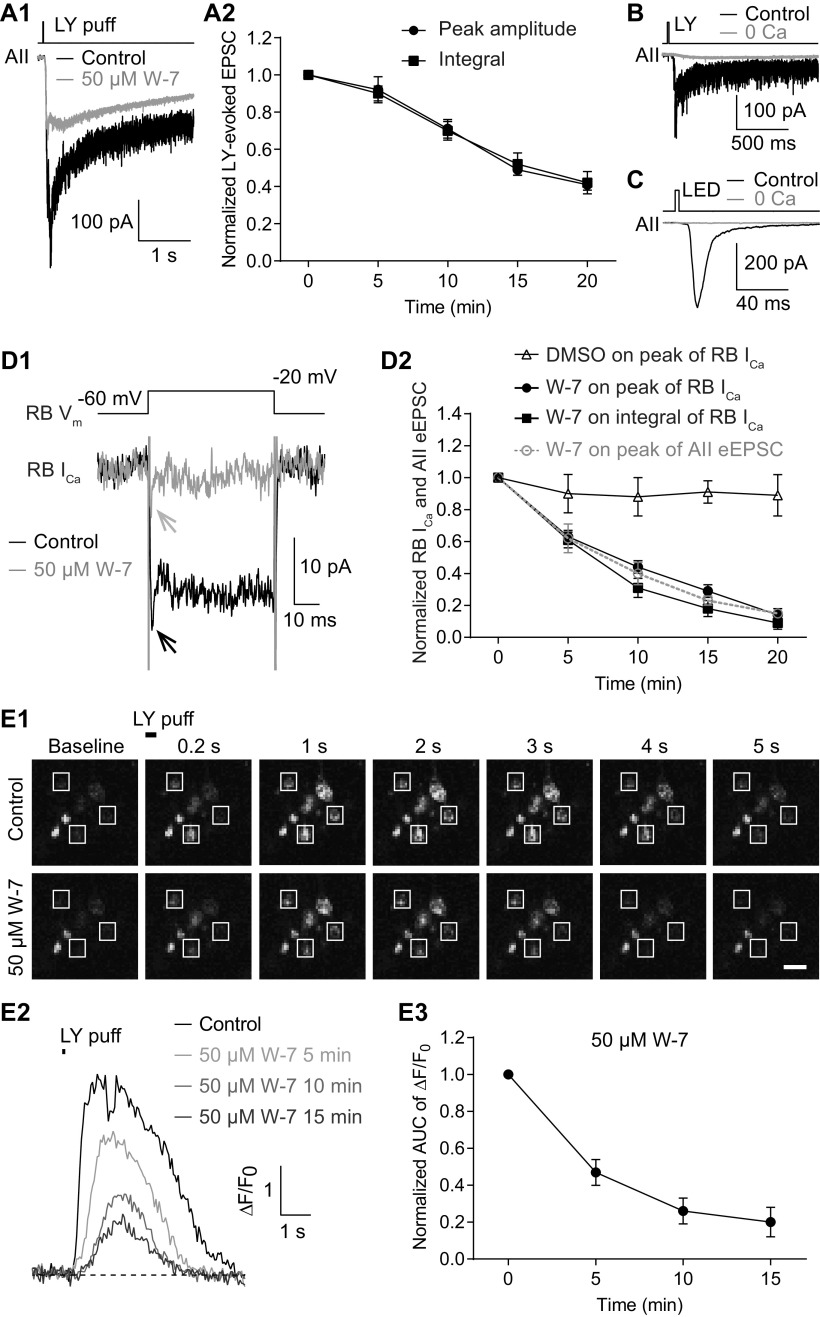
Inhibition of CaM strongly reduces evoked release from RBs by suppressing Ca2+ influx into their axon terminals. A1, Average traces showing that EPSCs recorded in an AII, evoked by puffing LY 341495 (LY), an mGluR6 antagonist, onto the dendrites of RBs located at the OPL, were strongly reduced by application of 50 μm W-7. Vhold = −80 mV. A2, LY-evoked EPSCs decreased over time with application of 50 μm W-7 (n = 7). The peak amplitudes and integrals of EPSCs were normalized to the amplitude and integral at time 0, respectively, in each cell before averaging across cells. B, LY-evoked EPSCs were completely abolished by removing extracellular Ca2+ (0 Ca). C, The EPSCs recorded in an AII, evoked by brief flashes of 470-nm LED in a Pcp2-cre::Ai32 mouse, were abolished completely by removing extracellular Ca2+ (0 Ca). D1, Average traces showing that W-7 (50 μm) strongly suppressed the voltage step-generated Ca currents (ICa) in an RB. D2, Statistics of the effects of 50 μm W-7 (n = 7) and DMSO control on the peak amplitude and integral of RB ICa. The suppression of ICa recorded in RBs was closely related to the inhibition of eEPSCs recorded in AIIs (data adapted from Fig. 2B3, superimposed in gray). E1, Calcium imaging pictures from a Pcp2-cre::Ai38 mouse showing that LY-evoked changes of Ca2+ signals in RB axon terminals (white frames), detected by the Ca2+ indicator GCaMP3, were reduced strongly by application of 50 μm W-7. Scale bar: 1 μm. E2, Representative ΔF/F0 traces showing suppression of Ca2+ signals in an RB axon terminal by 50 μm W-7. E3, Summary data showing that LY-evoked Ca2+ signals in RB axon terminals (n = 21 terminals from 3 retinal slices), measured as areas under the curve (AUCs) of ΔF/F0 traces, were strongly suppressed by 50 μm W-7 over time. All the data were illustrated as mean ± SEM.