Abstract
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are increasing in incidence. Clinicians urgently need a method that can effectively predict the prognosis of GEP-NENs.
A total of 14770 GEP-NENs patients with pathologically confirmed between 1975 and 2016 were obtained from the surveillance, epidemiology, and end results database. All the patients were divided into primary (n = 10377) and validation (n = 4393) cohorts based on the principle of random grouping. Multivariate Cox proportional hazards proportional hazards regression analysis was performed to evaluate predictors associated with overall survival, and a nomogram was constructed based on the primary cohort. An independent external validation cohort and comparison with the eighth edition American Joint Committee on Cancer TNM staging system were subsequently used to assess the predictive performance of the nomogram.
The multivariate Cox model indicated that age, tumour differentiation, and distant metastases were independent predictors associated with overall survival. With respect to the primary cohort, the nomogram exhibited better discriminatory power than the TNM classification (C-index: 0.821 vs 0.738). Discrimination was also superior to that of TNM classification for the validation cohort (C-index: 0.823 vs 0.738). The calibrated nomogram predicted 3- and 5-years survival rate that closely corresponded to the actual survival rate.
This study developed and validated a prognostic nomogram applied to patients with GEP-NENs, which may help clinicians make reasonable prognostic judgments and treatment plans to a certain extent.
Keywords: gastroenteropancreatic, neuroendocrine neoplasms, nomogram, overall survival, prediction
1. Introduction
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are the most common neuroendocrine tumors (62%–67%),[1] constitute a heterogeneous group of genetically diverse neoplasms arising from the secretory cells of the neuroendocrine system, with various clinical presentations and biological behaviors that current diagnostic and therapeutic challenges.[2] In recent years, the incidence of GEP-NENs has been increasing,[3,4] the reported annual age-adjusted incidence of neuroendocrine tumors was 1.09 per 100 000 persons in 1973 and increased to 6.98 per 100 000 persons by 2012 in the United States.[4] As a general rule, early-stage GEP-NETs are associated with a very favorable long-term prognosis,[5] however, GEP-NENs are often metastasized at the time of diagnosis and curative resection is not possible in all cases.[6] And it is difficult to predict the prognoses for patients with GEP-NENs,[7] with the heterogeneous and complex biological behaviour and ineffective treatment.
Prognostic staging of GEP-NENs is decisive in suggesting appropriate treatments, stratifying participants, and counselling patients on the severity of their disease. However, there is still a lack of high-quality prognostic risk evaluation models.[8] The TNM staging system of American Joint Committee on Cancer (AJCC) is 1 of the commonly used prognostic systems for GEP-NENs, however, its clinical utility has yet to be clinically verified.[8,9] The 2010 WHO classification categorizes the Ki-67 proliferative index and mitotic count as diagnostic and prognostic factors for GEP-NENs, although this classification system is simple and practical, it has been criticized on the basis that it does not reflect real disease status.[7] Furthermore, other demographics and clinical characteristics such as age at diagnosis, sex, tumor size, tumour location and microenvironment and tumor inflammatory features can also influence patient outcomes.[10,11]
The nomogram has been accepted as a reliable tool to create a simple intuitive graph for a predictive statistical model that quantifies the risk of a clinical event. Previous researches have successfully quantified the risk of certain cancers, by combining the key prognostic factors.[12–14] To data, few studies have used nomograms combining treatment and clinicopathological features to predict the prognosis of GEP-NENs patients. Our study aim to develop and validate a more elaborative nomogram to predict 3- and 5-year overall survival rates based on a relatively large cohort of patients with GEP-NENs from the surveillance, epidemiology, and end results (SEER) database.
2. Material and methods
2.1. Ethics approval and consent to participate
This research was exempted by the Ethics Committee of the Affiliated Tumor Hospital of Guangxi Medical University, because data extracted from the publicly available SEER database were recognized as nonhuman studies.[15]
2.2. Patients and data source
We identified GEP-NENs cases from SEER∗Stat software of the National Cancer Institute (Version 8.3.6).[16] Because information about the variable chemotherapy and radiotherapy, which indicated the treatment of GEP-NENs, was not available after 2016 years, patients diagnosed with GEP-NENs between 1975 and 2016 were finally included in our research, using the database, ‘Incidence – SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1973–2016 varying)’.
Cases of NENs were identified according to the International Classification of Diseases codes for Oncology (ICD-O-3) and the ‘Site recode ICD-O-3/WHO 2008’ data were used to select by tumour location. Patients with unknown follow-up and unclear tumor location were excluded, patients for whom GEP-NENs was not their first primary tumor were also excluded. All cases were uniformly reviewed and staged according to the 8th edition[17] of the AJCC TNM classification. Finally, a total of 14770 patients were enrolled and analysed in our study. According to the principle of random grouping, the patients are divided into the primary cohort (n = 10377) and the validation cohort (n = 4393) according to the ratio of 7:3. The variables sex, race, age at diagnosis, T staging, N staging, M staging, differentiation (the Ki-67 index was categorised as ‘well differentiated’, ‘moderately differentiated’, or ‘poorly differentiated/undifferentiated’ in the SEER database), tumour location, stage, marital status at diagnosis, primary tumor size, tumor location, radiation, chemotherapy, survival time, survival status were used in our research.
2.3. Statistical analysis
The statistical analyses were conducted with SPSS version 26.0 (IBM Corp, Armonk, NY), and graphics produced with R software (rms[18] and survival[19] in R version 3.6.2; https://www.r-project.org/). Continuous data are presented as median (interquartile range [IQR]) or mean ± standard deviation. Categorical data are presented as numbers and proportions. Differences between groups were evaluated by a chi-square test, a t-test, or the Wilcoxon rank-sum test. The 3- and 5- year's survival rate was calculated by the Kaplan-Meier method and the median follow-up time was calculated using the reverse Kaplan Meier estimator. The proportional hazards (PH) and linearity assumptions for the continuous variables (ie, age) were examined using restricted cubic splines. Then, the clinical significance of veriables in terms of overall survival (OS) in patients with GEP-NENs was further assessed by univariate and multivariate Cox PHs regression analyses without violating the PH assumption. All tests were 2 sided, and P < .05 were considered to be statistically significant. Finally, a prognosis nomogram for patients with GEP-NENs was constructed based on the results from the final multivariable Cox PHs regression.
Calibration curves were plotted to assess the calibration of the nomogram. The C-index was used to assess discriminative power of the nomogram, ranging from 0.5 (absence of discrimination) to 1 (perfect discrimination).[20,21] In addition, the nomogram was subjected to 1000 bootstrap resamples for internal validation to assess predictive accuracy.[21]
3. Results
3.1. Demographic and clinicopathological characteristics
A total of 14,770 patients with gastrointestinal pancreatic neuroendocrine tumors were included in the study from 1975 to 2016. The median patient age was 60 years (IQR, 51–70 years), and 49.6% of the patients were female. Most patients (78.2%) were of the white race. The most common tumor location is the small intestine (32.8%), followed by the pancreas (23.9%), cecum, rectum, stomach, colon and appendix accounted for 5.4%, 14.6%, 7.7%, 5.1%, and 10.5% respectively. Most tumors were early, with 39.0% being stage I tumors and 17.1% being stage II tumors. In total, 68.1% of patients’ tumors were well differentiated, with 17.1% and 14.9% being moderately differentiated and poorly differentiated respectively. The median follow-up time was 41.0 months and the median OS was 131 months, and the 3-, and 5-year OS rates were 77.8% and 70.8%, respectively.
10377 patients were involved in the primary cohort and 4393 patients were involved in the validation cohort. The demographic and clinicopathologic characteristics of patients in the primary and validation cohorts were listed in Table 1. The characteristics of the patients in the primary and external validation cohort were similar. In the primary cohort, the median follow-up time was 41.0 months. The median OS was 131 months, and the 3-, and 5-year OS rates were 78.0% and 70.8%. In the validation cohort, the median follow-up time was 40.0 months. The median OS was 142 months, and the 3-, and 5-year OS rates were 77.3% and 70.8%.
Table 1.
The demographic and clinicopathological characteristics of the primary and validation Cohorts.
| Variable | Primary Cohort | Validation Cohort | P-value |
| Age, yr | 60 (50,70) | 60 (50,70) | .754 |
| Race, N (%) | .525 | ||
| White | 8129 (78.3) | 3424 (77.9) | |
| Black | 1523 (14.7) | 639 (14.5) | |
| Other | 725 (7.0) | 330 (7.5) | |
| Male, N (%) | 5239 (50.5) | 2207 (50.2) | .783 |
| Differentiation, N (%) | .761 | ||
| Well | 7034 (67.8) | 3005 (68.4) | |
| Moderately | 1787 (17.2) | 741 (16.9) | |
| Poorly | 1556 (15.0) | 647 (14.7) | |
| Radiation, yes, N (%) | 209 (2.0) | 69 (1.6) | .040 |
| Chemotherapy, yes, N (%) | 1327 (12.8) | 583 (13.3) | .424 |
| Tumour location, N (%) | .813 | ||
| Cecum | 559 (5.5) | 234 (5.3) | |
| Small Intestine | 3430 (33.1) | 1413 (32.2) | |
| Pancreas | 2461 (23.7) | 1062 (24.2) | |
| Rectum | 1509 (14.5) | 650 (14.8) | |
| Stomach | 810 (7.8) | 327 (7.4) | |
| Colon | 528 (5.1) | 227 (5.2) | |
| Appendix | 1070 (10.3) | 480 (10.9) | |
| Stage, N (%) | .668 | ||
| Localized | 5014 (48.3) | 2117 (48.2) | |
| Distant | 2469 (23.8) | 1073 (24.4) | |
| Regional | 2894 (27.9) | 1203 (27.4) | |
| Tumour size | .109 | ||
| <2cm | 5514 (53.1) | 2273 (51.7) | |
| 2–4cm | 2923 (28.2) | 1236 (28.1) | |
| >4cm | 1940 (18.7) | 884 (20.1) | |
| T staging, N (%) | .394 | ||
| T0 | 63 (0.6) | 24 (0.5) | |
| T1 | 3816 (36.8) | 1602 (36.5) | |
| T2 | 2044 (19.7) | 859 (19.6) | |
| T3 | 2868 (27.6) | 1246 (28.4) | |
| T4 | 1392 (13.4) | 601 (13.7) | |
| Tx | 194 (1.9) | 61 (1.4) | |
| N staging, N (%) | .659 | ||
| N1 | 10314 (99.4) | 4369 (99.5) | |
| N0 | 63 (0.6) | 24 (0.5) | |
| M staging, N (%) | .919 | ||
| M1 | 2156 (20.8) | 916 (20.9) | |
| M0 | 8221 (79.2) | 3477 (79.1) | |
| Marital status at diagnosis | .574 | ||
| Single | 1928 (18.6) | 843 (19.2) | |
| Marry | 6444 (62.1) | 2674 (60.9) | |
| Widowed | 951 (9.2) | 413 (9.4) | |
| Divorced | 1054 (10.2) | 463 (10.5) | |
| Survival time, month | 32 (16,53) | 31 (16,52) | .066 |
| Survival status, dead, N (%) | 2559 (24.7) | 1080 (24.6) | .922 |
Other race: American Indian/AK Native, Asian/Pacific Islander.
3.2. Model specifications and predictors of OS
The results of the univariate and multivariate Cox PHs regression analysis were listed in Tables 2 and 3. Univariate analyses demonstrated that age, race, sex, differentiation, radiation, chemotherapy, stage, tumour size, T staging, M staging and marital status at diagnosis were associated with OS. Multivariate analysis demonstrated that age, sex, differentiation, chemotherapy, tumour size, T staging and M staging were independent risk factors for OS.
Table 2.
Univariate Cox PHs analysis showing the association of variables with overall survival rate in the primary cohort.
| Variable | HR | 95% CI | P-value |
| Age | 1.045 | 1.042–1.048 | <.001 |
| Race | 0.861 | 0.801–0.926 | <.001 |
| Sex | 0.786 | 0.727–0.850 | <.001 |
| Differentiation | 3.005 | 2.872–3.145 | <.001 |
| Radiation | 2.246 | 1.864–2.706 | <.001 |
| Chemotherapy | 4.346 | 4.003–4.718 | <.001 |
| Tumour location | 1.017 | 0.999–1.036 | .071 |
| Stage | 1.334 | 1.277–1.393 | <.001 |
| Tumour size | 2.360 | 2.249–2.476 | <.001 |
| T staging | 1.719 | 1.660–1.781 | <.001 |
| N staging | 1.057 | 0.647–1.729 | .824 |
| M staging | 4.407 | 4.077–4.763 | <.001 |
| Marital status at diagnosis | 1.185 | 1.132–1.239 | <.001 |
Table 3.
Selected variables according to the multivariate Cox PHs regression model based on the primary cohort.
| Variable | HR | 95% CI | P-value |
| Age | 1.039 | 1.036–1.042 | <.001 |
| Race | 1.042 | 0.970–1.118 | .262 |
| Sex | 0.808 | 0.747–0.873 | <.001 |
| Differentiation | 2.036 | 1.926–2.153 | <.001 |
| Radiation | 0.898 | 0.741–1.089 | .274 |
| Chemotherapy | 1.299 | 1.177–1.435 | <.001 |
| Stage | 1.034 | 0.974–1.097 | .273 |
| Tumour size | 1.265 | 1.191–1.344 | <.001 |
| T staging | 1.092 | 1.047–1.139 | <.001 |
| M staging | 2.602 | 2.384–2.841 | <.001 |
| Marital status at diagnosis | 1.022 | 0.972–1.074 | .401 |
3.3. Prognostic nomogram for OS and model performance in the primary cohort
Figure 1 illustrates the predictive nomogram established for the 3- and 5-year overall survival rates based on the selected parameters in the primary cohort. The model 1 was established that included 7 significant predictors for colorectal cancer prediction (Fig. 1A). The model 1 demonstrated moderate discrimination in predicting the OS of GEP-NENS, with an unadjusted C Index of 0.826 (95%Cl, 0.818–0.834), but the model 1 that combines 7 factors is cumbersome. Sex, chemotherapy, T staging, and tumor size have little influence on the point in the nomogram. We try to reduce the number of factors to simplify the nomogram for ease of use, finally found that the model 2 (Fig. 1B) includes factors with age, differentiation, and M staging still maintain good discrimination with 0.821 (95%Cl, 0.813–0.829), corrected to 0.820 via bootstrapping validation (B = 1000), which suggested good discrimination by our model 2. In addition, overall calibration plots were outstanding for the OS at 3 or 5 year between the probabilities predicted by the nomogram and actual probabilities (Fig. 2).
Figure 1.
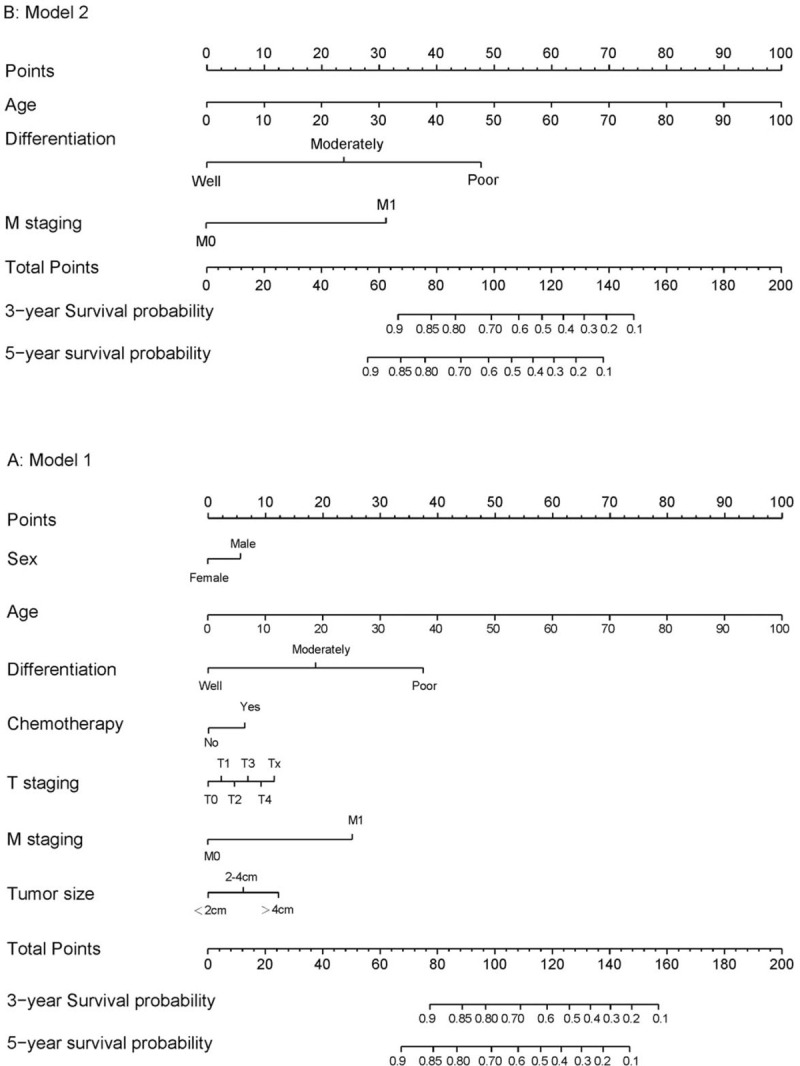
Nomogram Predicting Overall Survival in Patients with GEP-NENs. The nomogram finds the position of each variable on the corresponding axis, draws a line to the point's axis for the number of points, adds the points from all of the variables, and draws a line from the total point's axis to determine the colorectal cancer probabilities at the lower line of the nomogram.
Figure 2.
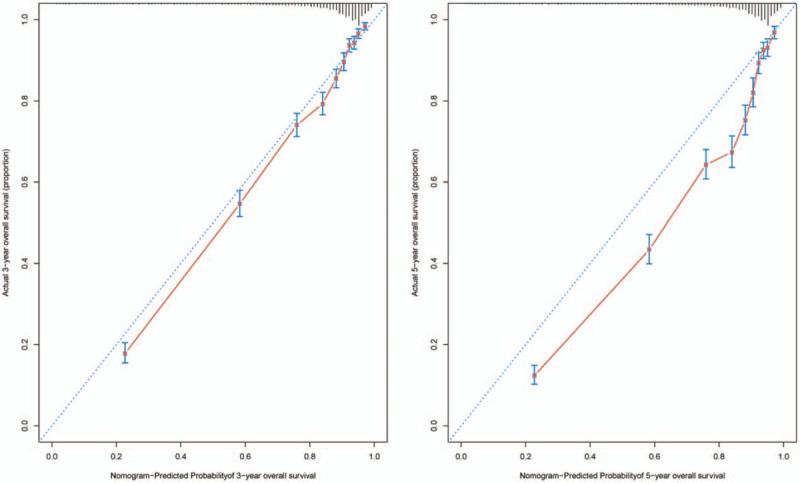
The calibration of the nomograms using the primary cohort set. The x axis represents the nomogram predicted survival rate, whereas the y axis represents the actual survival rate. (B = 1000).
3.4. External validation of the nomogram in the validation cohort
In the validation cohort, The C-index of the final nomogram for predicting OS was 0.823 (95%CI, 0.809 to 0.837), and a calibration curve showed overall satisfaction between prediction and observation in the probability of 3 or 5 year survival (Fig. 3).
Figure 3.
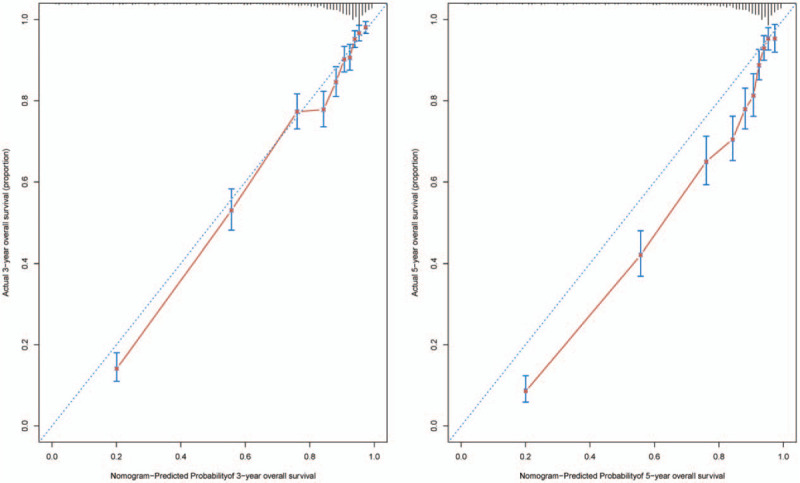
The calibration of the nomograms using the validation cohort set. The x axis represents the nomogram predicted survival rate, whereas the y axis represents the actual survival rate. (B = 1000).
3.5. Comparison of the predictive value of nomogram and eighth edition AJCC TNM staging
We compared the discrimination of the nomogram with that of the Eighth edition AJCC TNM classification in the primary cohort. The nomogram discrimination was superior to that of the Eighth edition AJCC TNM classification (C-index 0.821, 95%Cl = 0.813–0.829 vs 0.738, 95%CI = 0.729–0.748). Discrimination was also compared with the 8TH AJCC TNM staging with regard to the validation set (C-index 0.738, 95%CI = 0.729–0.748 vs 0.823, 95%CI = 0.809–0.837). The nomogram exhibited better survival predictive ability to that of the Eighth edition AJCC TNM staging system.
4. Discussion
Our study developed and validated a novel nomogram to predict the OS rate of patients with GEP-NENs based on the SEER database. This nomogram showed more significantly predictive than the eighth AJCC stage grouping, with C-index of 0.821 (95% CI = 0.813–0.829). The external validation also demonstrated that the nomogram showed overall excellent predictive ability compared with the TNM staging system, with C-index of 0.823 (95%CI = 0.809–0.837). The calibration plots verified that the predicted 3- and 5-year OS rates closely corresponded to the actual OS rates with regard to both the primary and validation cohort. It is worth mentioning that the tumor location is considered to be a major factor in the prognosis,[4,22] but the tumor location in our study did not show statistical significance related to OS. In view of the fact that GEP-NENs may not be reported to the cancer registry unless they are considered malignant, we may underestimate the true incidence and prevalence, leading to the loss of patients with benign tumors.
The incidence of GEP-NENs has significantly risen over recent decades, which may be related to the dyspepsia syndrome caused by cobalamin deficiency.[23] The improving diagnostic techniques and awareness also play an important role. However, prognosis of GEP-NENs is difficult to predict in clinical practice for their heterogeneous outcomes, choosing the appropriate treatment according to the patient's prognosis is still a big problem for the clinicians. The criteria for evaluating prognosis for GEP-NENs differ for distinct pathological differentiation statuses and clinical systems. There have been previous reports show the favorable predictive ability of nomograms for NENs with stomach,[24] liver metastases,[25] small intestine[26] and pancreas.[27] These results verify that a consistent and specific nomogram could be clinically applied to effectively and accurately predict the prognosis of patients with NENs. However, precedent studies contained limited patient data about radiotherapy and chemotherapy or lacked external validation. Our research can be taken up to overcome these limitations.
According to our nomogram, the disease-specific mortality risk prediction increases with age, and the median age at dignosis of patients was 60 years, which confirmed prior findings of the prognostic significance of age at diagnosis.[4,28] In addition, our nomogram demonstrates the magnitude of poor prognosis as the tumor differentiation poorer. Our nomogram also clearly illustrates that patients with distant metastases are more likely to die than those without metastases, which reveals an interesting phenomenon: the classic N stage failed to show an independent prognostic significance. The TNM staging system can provide important prognostic values for other types of tumours, however, it seems that the application value in neuroendocrine tumors has not been universally recognized. Jacob A et al[9] found that multiple stages, determined by current criteria of the AJCCTNM staging system, misclassified patients’ prognosis. Several groups have also assessed the prognostic accuracy of current GEP-NENs staging and found that both the American and European TNM classifications have overlap stages and contain considerable variability in survival.[29–31] However, currently Ki-67 does not have a clear cut-off value, and the grade of differentiation may vary due to different geographical factors.[32]
Clinicians could recommend certain admonitions based on the total risk calculated by the nomogram. For example, the AJCC guidelines recommend that patients with poor differentiation would receive palliative chemotherapy or participate in clinical trials for their short life expectancy. However, selecting patients based solely on TNM classification may be ambiguous, and doctors will have to depend on their clinical experience. However, clinicians may be more accurate in selecting patients with a better survival rate for they would bear a higher probability of benefiting from treatments, by the nomogram consisting of clinicopathological factors.
Some limitations of the study should be noted. First, although in the current study, the parameters of sex, chemotherapy, T staging, and tumor size showed statistical significance related to OS, but they have little influence on the point in the nomogram. We assume that prognostic differences across sex, chemotherapy, T staging, and tumor size are subtle among patients with GEP-NENs, this may be related to differences in specific populations and geographic locations, however, our model provided a common predictive tool for patients with GEP-NENs. Second, the SEER database doesn’t provide information about the functional status of the GEP-NENs that may also affect survival. Third, the tumor location in our study did not show statistical significance related to OS. Theoretically, different tumor locations may represent different biological origins, however, our results failed to clarify this possibility. Finally, treatment factors, such as quality of surgery, specific radiotherapy and chemotherapy, were unavailable and may have confounded the results. Such disadvantage is natural of any retrospective, population-based research and may enhance concerns about the steady of the results. However, in the absence of high-quality GEP-NENs prognostic tools, our predictive model may help clinicians make reasonable prognostic judgments and treatment plans to a certain extent.
5. Conclusions
This study developed and validated a prognostic nomogram applied to patients with GEP-NENs, which may help clinicians make reasonable prognostic judgments and treatment plans to a certain extent. However, further validation using external data is necessary in order to promote the applicability of our nomogram in clinical practice.
Author contributions
Conceptualization: Si Xie, Xiaotong Wang, Lequn Li.
Data curation: Lei Li.
Formal analysis: Si Xie, Lei Li, Lequn Li.
Investigation: Si Xie, Xiaotong Wang.
Methodology: Si Xie, Lequn Li.
Software: Si Xie, Lei Li.
Validation: Si Xie, Xiaotong Wang, Lequn Li.
Writing – original draft: Si Xie.
Writing – review & editing: Lequn Li.
Glossary
Abbreviations: AJCC = American Joint Committee on Cancer, GEP-NENs = gastroenteropancreatic neuroendocrine neoplasms, OS = overall survival, PH = proportional hazards, SEER = surveillance, epidemiology, and end results.
References
- [1].Oronsky B, Ma PC, Morgensztern D, et al. Nothing But NET: a review of neuroendocrine tumors and carcinomas. Neoplasia (New York, NY) 2017;19:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang Y, Wang W, Jin K, et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett 2017;13:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gosain R, Mukherjee S, Ball S, et al. Geographic and demographic features of neuroendocrine tumors in the United States of America: a population-based study. Cancer 2020;126:792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence,;1; and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 2018;68:471–87. [DOI] [PubMed] [Google Scholar]
- [6].Aristizabal Prada ET, Auernhammer CJ. Targeted therapy of gastroenteropancreatic neuroendocrine tumours: preclinical strategies and future targets. Endocr Connect 2018;7:R1–r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohmoto A, Rokutan H, Yachida S. Pancreatic neuroendocrine neoplasms: basic biology, current treatment strategies and prospects for the future. Int J Mol Sci 2017;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen L, Zhou Z, Chen J. Interpretation and evaluation of the American Joint committee on Cancer (AJCC) 8th Edition Staging System for patients with gastroenteropancreatic neuroendocrine tumors. Chin J Gastrointest Surg 2017;20:972–6. [PubMed] [Google Scholar]
- [9].Martin JA, Warner RR, Wisnivesky JP, et al. Improving survival prognostication of gastroenteropancreatic neuroendocrine neoplasms: Revised staging criteria. Eur J Cancer 2017;76:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang M, Zhao P, Shi X, et al. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single-centre study. BMC Endocr Disord 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Milione M, Miceli R, Barretta F, et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J Pathol Clin Res 2019;5:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Feng LH, Su T, Bu KP, et al. A clinical prediction nomogram to assess risk of colorectal cancer among patients with type 2 diabetes. Sci Rep 2020;10:14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang Y-q, Liang C-h, He L, et al. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol 2016;34:2157–64. [DOI] [PubMed] [Google Scholar]
- [14].Su J, Miao LF, Ye XH, et al. Development of prognostic signature and nomogram for patients with breast cancer. Medicine (Baltimore) 2019;98:e14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ye G, Wang L, Hu Z, et al. Risk and prognostic nomograms for hepatocellular carcinoma with newly-diagnosed pulmonary metastasis using SEER data. Peer J 2019;7:e7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Surveillance Research Program NCI. SEER∗Stat software Available at: http://www.seer.cancer.gov/seerstat. 2020. [Google Scholar]
- [17].Amin MB, Greene FL, Edge SB, et al. AJCC Cancer Staging Manual [M]. 8th ed.New York: Springer; 2016. [Google Scholar]
- [18].Jr FEH. rms: Regression Modeling Strategies. R package version 6.0-0. Available at: https://CRAN.R-project.org/package=rms. 2020. [Google Scholar]
- [19].A Package for Survival Analysis in R. R package version 3.2-3, Available at: <URL: https://CRAN.R-project.org/package=survival>. 2020. [Google Scholar]
- [20].Harrell FE, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. Jama 1982;247:2543–6. [PubMed] [Google Scholar]
- [21].Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 2017;318:1377–84. [DOI] [PubMed] [Google Scholar]
- [22].Fang C, Wang W, Feng X, et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer 2017;117:1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Troilo A, Mecili M, Ciobanu E, et al. Oral vitamin B12: Efficacy and safety data in 31 patients with pernicious anemia and food-cobalamin malabsorption. Presse Med 2010;39:e273–9. [DOI] [PubMed] [Google Scholar]
- [24].Carmona-Bayonas A, Jiménez-Fonseca P, Lamarca Á, et al. Prediction of progression-free survival in patients with advanced, well-differentiated, neuroendocrine tumors being treated with a somatostatin analog: the GETNE-TRASGU study. J Clin Oncol 2019;37:2571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lv Y, Han X, Xu XF, et al. Risk factors affecting prognosis in metachronous liver metastases from WHO classification G1 and G2 gastroenteropancreatic neuroendocrine tumors after initial R0 surgical resection. BMC Cancer 2019;19:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kelly S, Aalberg J, Kim MK, et al. A predictive nomogram for small intestine neuroendocrine tumors. Pancreas 2020;49:524–8. [DOI] [PubMed] [Google Scholar]
- [27].Kelly S, Aalberg J, Agathis A, et al. Predicting survival of small intestine neuroendocrine tumors: experience from a major referral center. Pancreas 2019;48:514–8. [DOI] [PubMed] [Google Scholar]
- [28].Tai WM, Tan SH, Tan DMY, et al. Clinicopathologic characteristics and survival of patients with gastroenteropancreatic neuroendocrine neoplasm in a multi-ethnic asian institution. Neuroendocrinology 2019;108:265–77. [DOI] [PubMed] [Google Scholar]
- [29].Luo G, Javed A, Strosberg JR, et al. Modified staging classification for pancreatic neuroendocrine tumors on the basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J Clin Oncol 2017;35:274–80. [DOI] [PubMed] [Google Scholar]
- [30].Yang M, Zeng L, Yao WQ, et al. A comprehensive validation of the novel 8th edition of American Joint Committee on Cancer staging manual for the long-term survivals of patients with non-functional pancreatic neuroendocrine neoplasms. Medicine (Baltimore) 2020;99:e22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee L, Ito T, Jensen RT. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: recent advances and controversies. Expert Rev Anticancer Ther 2019;19:1029–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch 2018;472:341–9. [DOI] [PubMed] [Google Scholar]


