Fig. 2 |. Viral strategies for accessing neurons by virtue of their connectivity.
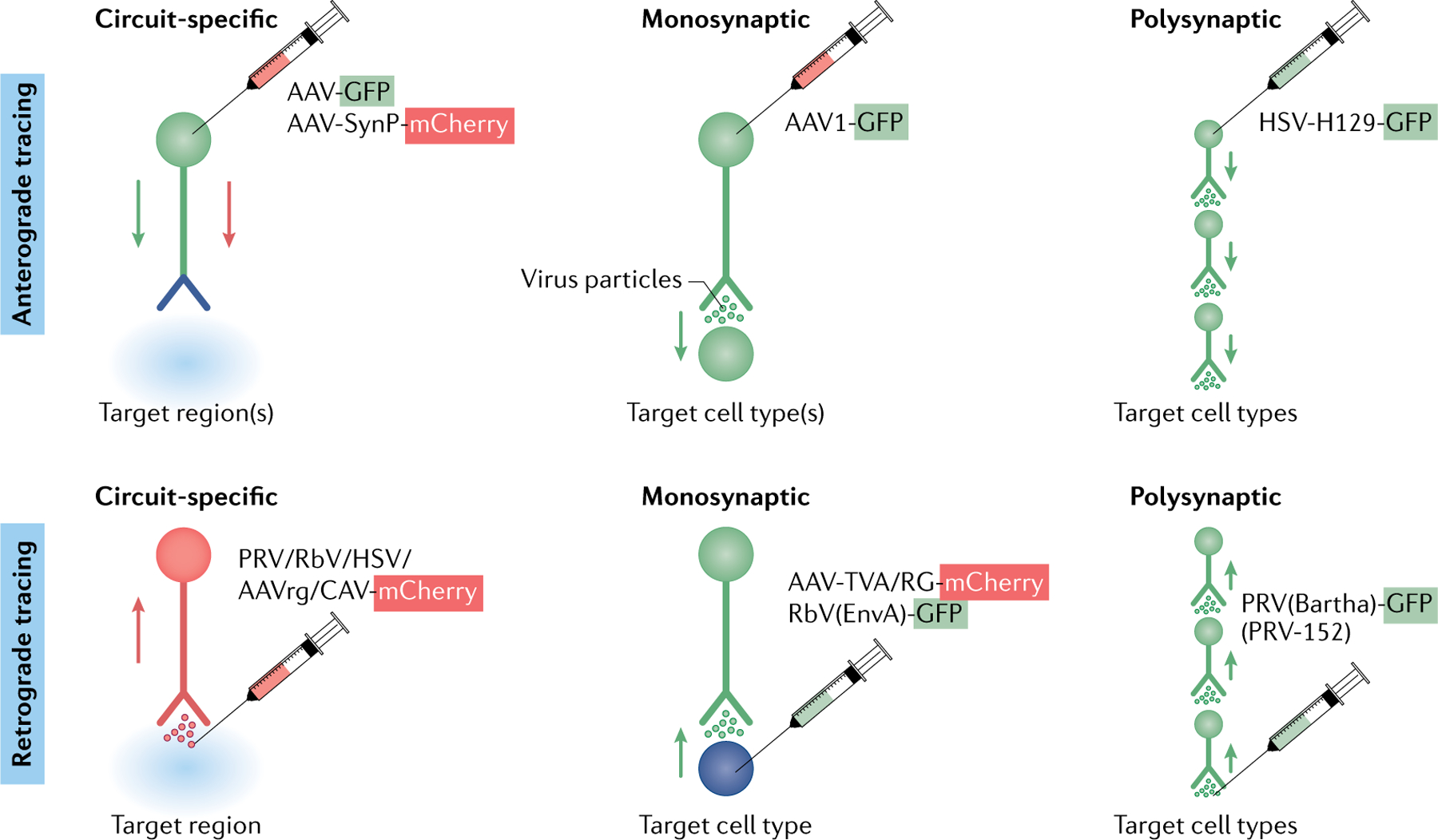
Viral tracing strategies commonly used to gain circuit-specific genetic access to cell types throughout the central nervous system. Tracing methods are commonly divided into anterograde (top) and retrograde (bottom) approaches, which often start by the injection of virus (indicated by a syringe) at a presynaptic soma or at the terminal or postsynaptic soma, respectively. Circuit-specific viral tracing methods most often utilize ‘gutted’ constructs (that is, they express few or no viral genes), which enable access to the cell type with limited toxicity. Monosynaptic and polysynaptic methods for accessing cell types are often, but not always, toxic to infected cells due to the frequent use of live, replication-competent viruses, which are capable of jumping one (monosynaptic) or more (polysynaptic) synapses. Green cell compartments are positive for GFP (green fluorescent protein), and red cell compartments are positive for mCherry (a monomeric, red fluorescent protein). Blue cell compartments (nerve terminals for circuit-specific anterograde tracing and postsynaptic soma in monosynaptic retrograde tracing) are positive for both GFP and mCherry. For circuit-specific anterograde tracing, AAV-GFP and AAV-SynP-mCherry, respectively, help to label the whole cell and the nerve terminals. Note that there is currently no way to access cells that are a given number of synapses upstream or downstream of a target cell type. AAV, adeno-associated virus (AAV1 is a variant of AAV); AAVrg, retrograde-tracing variant of AAV; CAV, canine adenovirus; EnvA, avian ASLV type A envelope protein (cognate ligand for TVA); HSV, herpes simplex virus; HSV-H129, anterograde-tracing variant of HSV; PRV, pseudorabies virus; RbV, rabies virus; RG, rabies G protein; SynP, a synaptophysin fusion construct (can target fluorophores to the presynaptic nerve terminal); TVA, avian receptor for EnvA.
