Fig. 3 |. Assessing a gene’s function within the CNS.
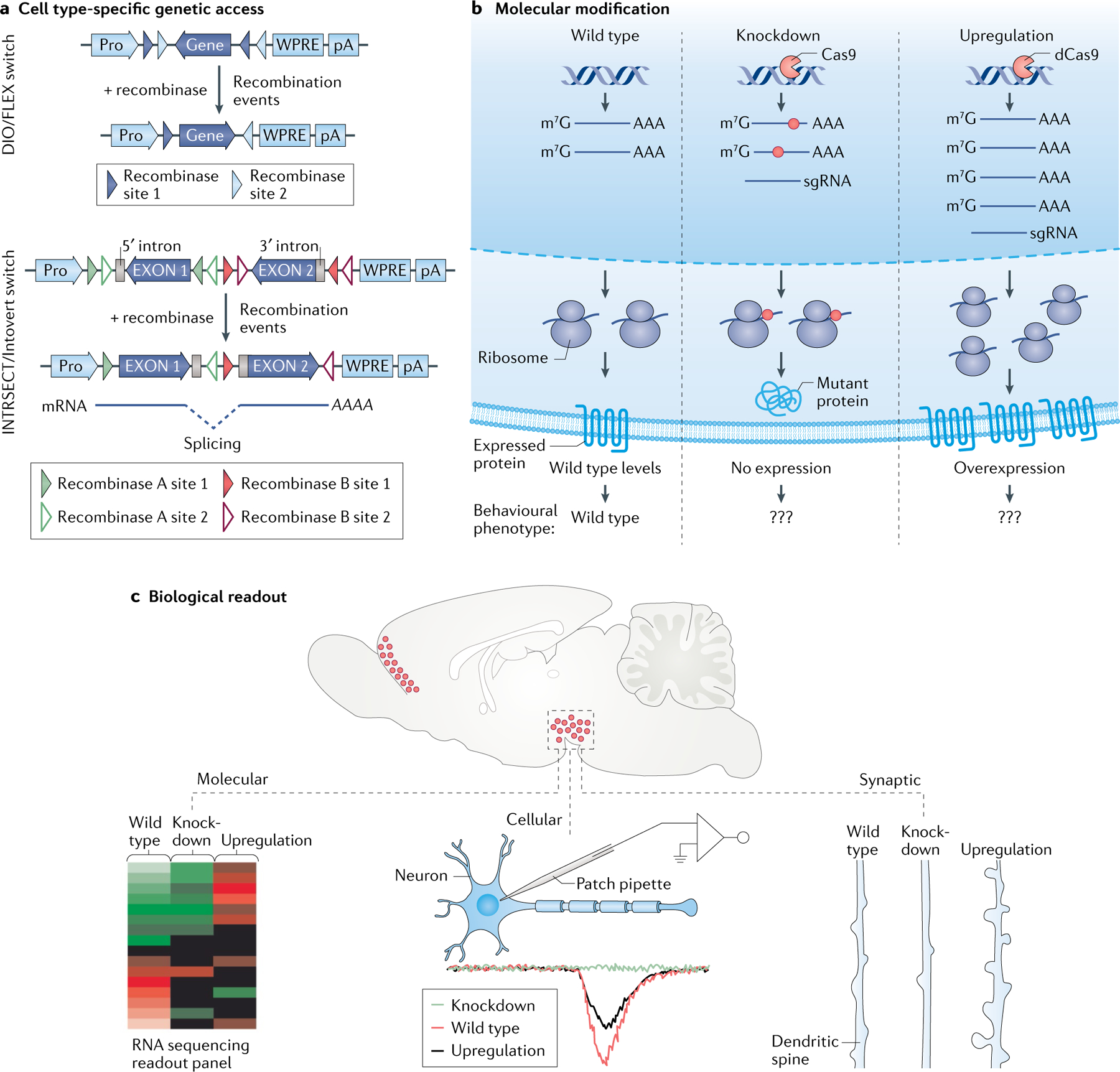
Theoretical framework for characterizing the role of a given gene in regulating molecular, cellular, synaptic, circuit and behavioural functions. a | To gain genetic access to a cell population in a cell type-specific fashion, single-recombinase (top) or dual-recombinase (bottom) ‘molecular logic’ can be used. The double-floxed inverted open reading frame (DIO)/flip-excision (FLEX; an irreversible, Cre-dependent molecular switch), often placed before a constitutive promoter (pro), allows for irreversible activation/expression of a cassette. When paired with cell type-specific expression of a recombinase (that is, Cre, Flp, Dre, VCre and so on) using a driver line, the DIO/FLEX switch is irreversibly activated, which leads to constitutive expression of the engineered transgene. This system thus benefits from the specificity of recombinase driver lines and the robust expression of constitutively active promoters (top). INTRSECT (a variant of the FLEX switch; INTRSECT constructs enable intersectional molecular logic through strategic use of introns) and Introvert (a variant of the FLEX switch, using a similar strategy to that of INTRSECT by strategically using introns)-based approaches can also be used to access a cell type using dual-recombinase logic (that is, based on the expression of two recombinases). These recombinases can be used to define any number of features of a cell population, allowing for increased specificity when genetically accessing a cell type (recombinases can be used to access a cell type based on marker gene expression, projection target or activity pattern). Introns are used in the INTRSECT/Introvert approaches to enable strategic inversions of split gene segments (so that the recombinase sites can be spliced out after recombination). This allows for the expansion of new, orthogonal molecular logic operations. Recombinase sites depicted are capable of recombining with one another only if they are of the same colour and shading (bottom). b | Molecular schemata for manipulations of gene expression. Among many options, a gene can be knocked down at the levels of DNA (using, for example, clustered regularly interspaced short palindromic repeats (CRISPR)) or RNA (microRNAs, newer RNA-targeting CRISPR systems). In the example shown, Cas9-mediated CRISPR knockdown is achieved via induction of an insertion-deletion in the genome. This genetic lesion is consequently manifested at the RNA level (represented in the middle sub-panel by a red dot), ultimately resulting in the formation of mutant (non-functional/inactive) protein. For upregulation of endogenous gene expression, a ‘dead’ Cas9 (dCas9) fusion construct enables increased transcription and, ultimately, more protein (represented in the right sub-panel). Triple question marks (???) represent the behavioural phenotype outcome being measured after genetic manipulations. c | Functional readouts after manipulating gene expression. Examples include gene expression adaptations revealed by RNA sequencing. This approach provides a readout of relative expression levels of wild-type, knockdown and upregulated genes (left). Alterations in synaptic strength can be determined using electrophysiology. The sample trace shows hypothetical responses from a neuron with a knockdown, wild-type or overexpressed gene product (middle). At the synaptic level, changes in dendritic spine number and morphology can be compared. These micro-scale adaptations may ultimately lead to observable changes in the animal’s circuit function and behaviour (right). AAA, 3′ poly(A) tail for mRNA; m7G, 7-methylguanosine (5′ cap for mRNA); sgRNA, single guide RNA (enables programmatic targeting of Cas proteins to a desired nucleic acid sequence); WPRE, woodchuck hepatitis virus post-transcriptional regulatory element.
