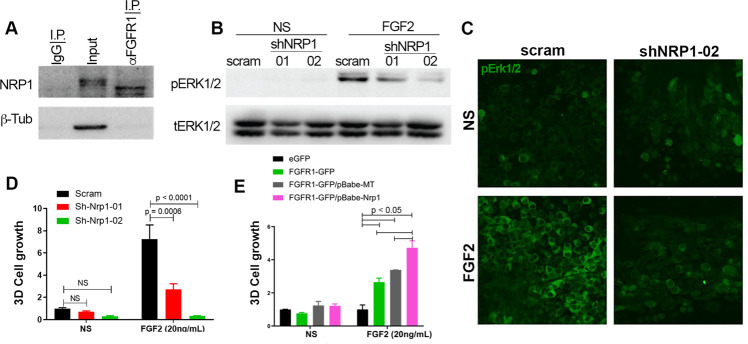
Fig. 5. NRP1 facilitates FGF2-mediated signaling and cell growth.
a Endogenous FGFR1 was immunoprecipitated from D2.A1 whole-cell lysates. These precipitates were probed by immunoblot with antibodies for NRP1. Total levels of NRP1 (input) and use of a non-specific antibody (IgG) are shown as controls. b Immunoblot analyses showing differential phosphorylation of ERK1/2 (pERK1/2) upon FGF2 (20 ng/ml) stimulation in control (scram) and NRP1-depleted (shNRP1-01 and -02) D2.A1 cells. Expression of total ERK1/2 (tERK1/2) served as a loading control. c Immunofluorescent staining for phosphorylated-ERK1/2 (pERK1/2) in control (scram) and NRP1-depleted (shNRP1-02) D2.A1 cells grown on 3D culture scaffolds. Cells were not stimulated (NS) or stimulated with FGF2 (20 ng/ml) for 10 min. Data in a–c are representative of at least three independent analyses. d Bioluminescent quantification of control (scram) and NRP1-depleted (shNRP1) D2.A1 cells growing under 3D culture conditions in the presence or absence (NS) of FGF2 (20 ng/ml). e Bioluminescent quantification of NMuMG cells stably expressing control vectors (eGFP and empty (MT)) or FGFR1-GFP and NRP1 in 3D culture in the presence or absence (NS) of FGF2 (20 ng/ml). For d, e, readings were taken 6 days after plating and are the mean ± SE of two independent experiments completed in triplicate resulting in the indicated P values.