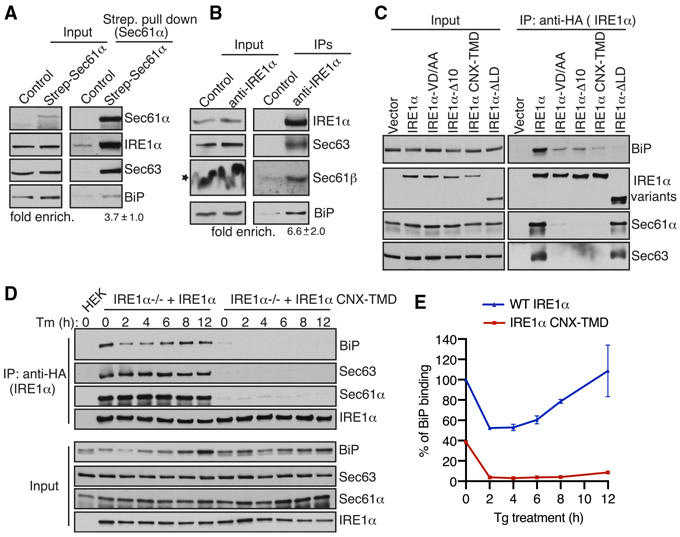
Figure 4. IRE1α Binding to BiP Strictly Depends on Its Interaction with Sec61/Sec63 in Cells.
(A) Control HEK293 cells or chromosomal 2xStrep-Sec61α HEK293 cells were lysed and affinity purified using Strep-Tactin beads. The bound proteins were eluted and analyzed by immunoblotting for the indicated antigens. Fold enrichment of BiP (mean ± SEM; n = 3; **p < 0.01 by Student’s t test) over the control was provided underneath the immunoblot.
(B) HEK293 cells were lysed and immunoprecipitated using either control anti-His antibodies or anti-IRE1α antibodies. The immunoprecipitates were analyzed by immunoblotting for the indicated antigens. Fold enrichment of BiP (mean ± SEM; n = 3; *p< 0.05 by Student’s t test) over the control is provided underneath the immunoblot. Star indicates that the distortion of the Sec61β band in the input was caused by co-migration with a high concentration of digitonin in the lysis buffer.
(C) HEK293 cells were transiently transfected with either IRE1α-HA or its variants, immunoprecipitated using an anti-HA antibody, and analyzed by immunoblotting.
(D) HEK293 IRE1α−/− cells stably expressing IRE1α-HA or IRE1α-CNX-TMD-HA were induced with 5 ng/mL doxycycline and left either untreated or treated with 10 μg/mL Tm for the indicated time points. The treated cells were harvested, lysed, and subjected to immunoprecipitation using anti-HA magnetic beads. The immunoprecipitates were analyzed by immunoblotting for the indicated antigens.
(E) Quantification results from IRE1α binding to BiP in (D). Error bars represent SEM from two independent experiments. WT IRE1α binding to BiP in unstressed cells was set as 100%.
Further data supporting these results are in Figure S7. See also Figure S5.