Figure 3.
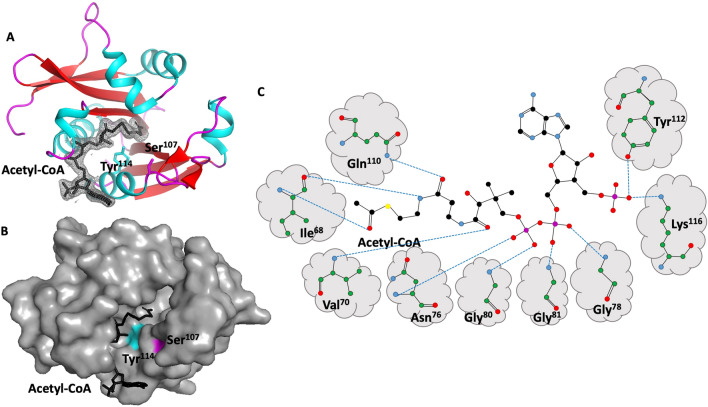
Acetyl-CoA binding site depicted within the tertiary structure the GNAT acetyltransferase from Elizabethkingia anophelis. Top left, shown in cartoon format, α-helices, β-strands, and loops are shown in cyan, red, and magenta, respectively. Acetyl-CoA is depicted in stick mode in black, with associated electron density map (2Fo-Fc) contoured at 1.0σ. The side chains of active site residues Tyr114 and Ser107 are depicted in stick mode. Bottom left, the same structure as above, but shown in surface view and coloured grey to depict the binding cleft. The location of Tyr114 and Ser107 are coloured cyan and magenta respectively. Right panel, schematic of hydrogen bond interactions between GNAT acetyltransferase residues and acetyl-CoA.