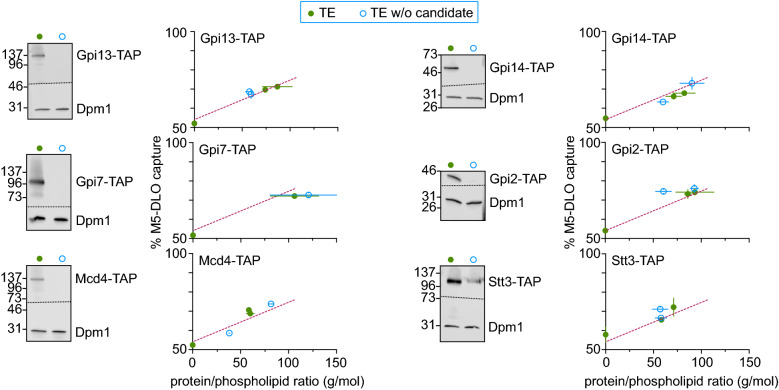
Figure 4.
Evaluating M5-DLO scramblase candidates by immunodepletion. TE was prepared from yeast strains expressing a C-terminally TAP-tagged variant of the scramblase candidate in place of the endogenous protein. Tagging was done at the genomic locus. Six unique TE preparations were thus generated, corresponding to cells expressing Gpi13-TAP, Gpi7-TAP, Mcd4-TAP, Gpi14-TAP, Gpi2-TAP or Stt3-TAP. In every case the extract was mock-treated (green filled circles) or incubated with IgG-agarose beads to remove the TAP-tagged protein (blue open circles). Removal of the TAP-tagged protein from the extract was confirmed by SDS-PAGE/immunoblotting using anti-TAP antibodies, and specificity of immunodepletion and equivalence of sample loading was confirmed by immunoblotting to detect the ER resident membrane protein Dpm1. Mock-treated and depleted extracts were reconstituted and assayed for M5-DLO scramblase activity. Reconstitutions were performed at different ratios of protein to phospholipid, in the approximate range 25–125 g/mol. The figure shows immunoblots paired with the corresponding activity assays for each candidate. Error bars are obtained from duplicate measurements of activity, as well as duplicate measurements of the protein and phospholipid content of the reconstituted vesicles. The red dashed line, based on all data from the mock-treated samples, is intended to guide the eye. See Fig. S5 for uncropped versions of the immunoblots.