Figure 7. Rabl2Q80L knock‐in mice display neonatal death and duplex kidney that phenocopy Ift27‐deficient mice.
-
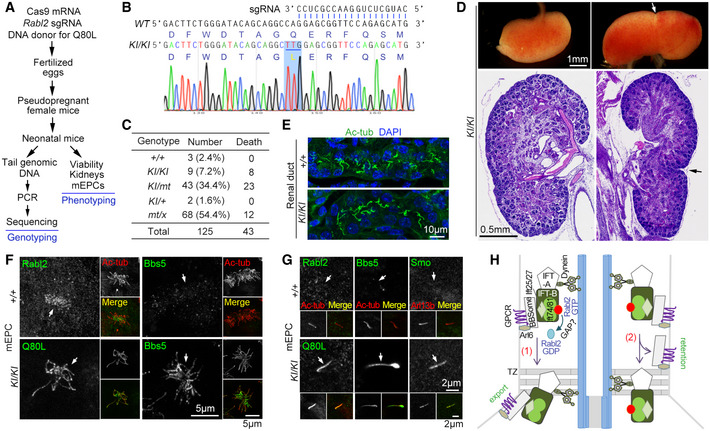
AExperimental scheme for the knock‐in (KI) mice production and characterization. The single‐stranded DNA donor‐mediated repair of the Cas9‐induced Rabl2 DNA breaks is expected to introduce the Q80L mutation in fertilized eggs or early embryos. Neonatal F1 mice were obtained through the cesarean section at E18.5. Genotyping was performed by directly sequencing the PCR products that covered the expected Cas9‐cleavage region of Rabl2.
-
BRepresentative sequencing results for Rabl2KI / KI mice, shown with corresponding sequences for wildtype Rabl2 and the 3′ end of the sgRNA. The TTG codon for the Q80L mutation is underlined.
-
CViability of neonatal mice in the day of birth after the cesarean section at E18.5. +, wildtype allele of Rabl2; KI, knock‐in allele with Q80L mutation; mt, allele with Cas9‐induced mutation; x, ambiguous allele. Refer to Fig EV5 for additional genotyping results. As these "heterozygous" mice might contain chimeric ones, the putative genotypes are used only for simplicity.
-
DMorphologically normal and duplex kidneys of Rabl2KI / KI mice. The whole kidneys were from a single mouse. The histological sections, stained with hematoxylin and eosin, were from different mice. Arrows point to the constriction of the duplex kidneys.
-
ERenal ducts of Rabl2KI / KI kidney displayed normal ciliary formation as compared to wildtype kidney. Nuclear DNA was stained with DAPI.
-
F, GEndogenous Rabl2Q80L entered ciliary shaft to repress the export of the BBSome (Bbs5) and its cargo GPCR (Smo). mEPCs cultured from Rabl2KI / KI and wild‐type neonatal mice were fixed at day 7 post‐serum starvation. Anti‐Rabl2 antibody was used to visualize Rabl2 and Rabl2Q80L. Ac‐tub or Arl13b served as ciliary marker. Representative micrographs of multicilia (F) and primary cilia (G) are shown. The white arrows point to positions of cilia.
-
HA model delineating the switch role of ciliary Rabl2 in the BBSome‐mediated GPCR turnover: (1) GTP hydrolysis induced presumably by a GTPase‐activating protein (GAP) dissociates Rabl2 from IFT‐B to enable a tight BBSome‐IFT‐B interaction in the retrograde IFT machinery for concomitant TZ passage; (2) Without the GTP hydrolysis, Rabl2‐GTP (or Rabl2Q80L) binds persistently to IFT‐B, resulting in shedding of the BBSome and its cargos from the IFT machinery in the TZ passing process and accordingly their ciliary retention. See Discussion for details. For simplicity, kinesin motor is not illustrated.
