Fig. 6. Sustainability of protein glycosylation by the TRAP complex during ER stress.
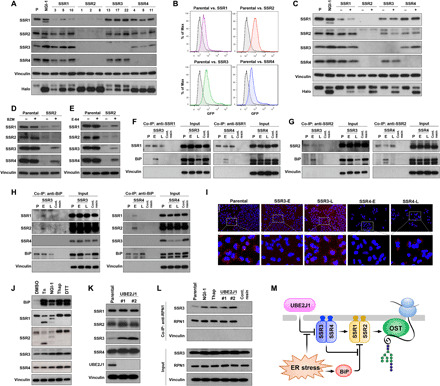
(A) TRAP subunit protein levels for independent A549 clones with SSR1, SSR2, SSR3, or SSR4 KO. (B) FACS and (C) Western blots of HEK-293–Halo1N-Cas9 with individual subunit KO. (D) TRAP subunit protein levels in parental or SSR2 KO cells treated with the proteasome inhibitor bortezomib (BZM; 100 nM) for 24 hours or (E) the cysteine peptidase inhibitor, E-64 (10 μM for 24 hours). (F to H) Co-immunoprecipitation (Co-IP) of BiP in A549-SSR3 KO or SSR4 KO cells using (F) anti-SSR1, (G) anti-SSR2, and (H) anti-BiP. (I) SSR1 and SSR2 interactions determined by the proximity ligation assay in parental, SSR3, and SSR4 early (E) or late (L) cells (scale bar, 100 μm). (J) TRAP subunit levels after ER stress activation by [tunicamycin (Tn), NGI-1, thapsigargin (Thap), and dithiothreitol (DTT)]. (K) TRAP subunit expression in UBE2J1 KO clones compared to the control cells. (L) Co-IP of SSR3 with RPN1 in Tn, NGI-1, Thap, and UBE2J1 KO cells. Control resin is used as the nonspecific binding control and vinculin is used as the negative interaction control. (M) Systems model of the functional roles for TRAP subunits in regulating N-glycosylation during ER stress.
