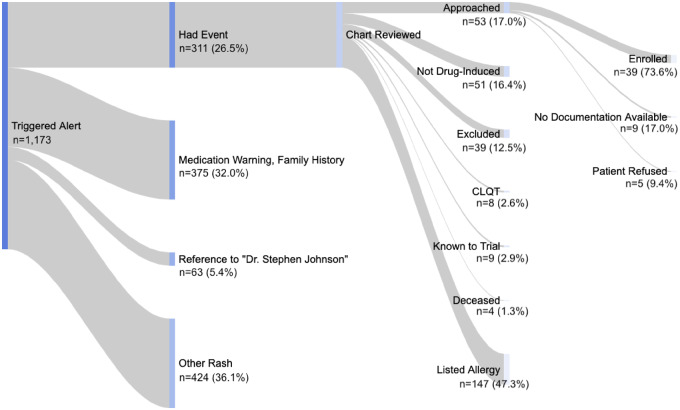
Figure 3.
Patients with adverse drug events enrolled by email text match. Total email alerts shown for unique medical record numbers (n = 1173). Contextual mentions classified by REDCap users and confirmed by manual chart review. Major exclusion criteria consisted of significant comorbidities (eg, history of bone marrow transplant) and age less than 18 years. Both studies required documentation (eg, rhythm strip) and event onset ≤4 weeks of initiation of drug. Had event indicated patients with a confirmed history of the adverse drug events drug-induced torsades de pointes or Stevens-Johnson syndrome and toxic epidermal necrolysis. Other rash indicates text mentions included as part of a differential diagnosis (n = 171) or negated (n = 253). CLQT: congenital long QT syndrome.