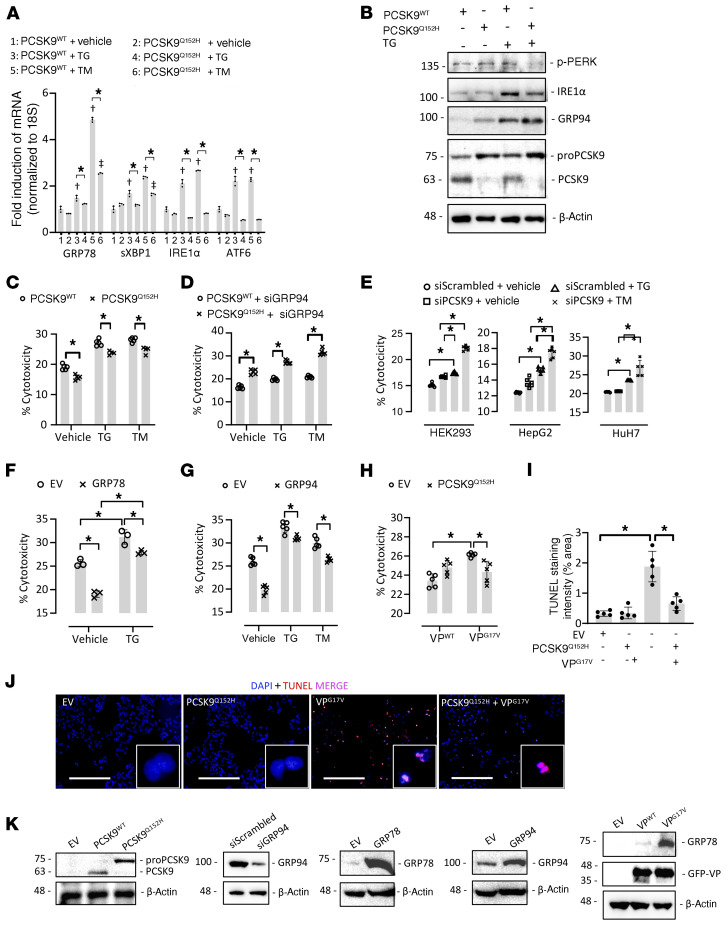
Figure 5. ER PCSK9 retention reduces ER stress–induced cytotoxicity in cultured immortalized hepatocytes.
(A) HEK293 cells were transfected with plasmids encoding either human PCSK9 WT (PCSK9WT) or the ER-retained variant PCSK9Q152H for 48 hours and subsequently treated with the ER stress–inducing agent thapsigargin (TG; 100 nM) or tunicamycin (TM; 2 μg/mL) for an additional 24 hours. ER stress marker expression was assessed by real-time PCR. (B) The expression of ER stress markers p-PERK, IRE1α, and GRP94 was also assessed in HuH7 immortalized hepatocytes transfected with either PCSK9WT or PCSK9Q152H in the presence and absence of TG using immunoblots. (C and D) Cytotoxicity was examined in PCSK9-transfected HepG2 cells in the absence (C) or presence (D) of siRNA targeted against GRP94 (siGRP94) using an LDH release assay. (E) Cytotoxicity was also examined in HEK293, HuH7, and HepG2 cells transfected with siRNA targeted against PCSK9 (siPCSK9) in the presence and absence of TG. (F and G) The effect of increased GRP78 and GRP94 expression on cytotoxicity caused by ER stress–inducing agents TG (100 nM) and TM (2 μg/mL) was also examined. (H) LDH release assays were carried out in HepG2 cells cotransfected with WT arginine vasopressin (VP) or a naturally occurring variant known to cause ER stress and cytotoxicity (VPG17V) in the presence or absence of ER-retained PCSK9Q152H. (I and J) These findings were confirmed by staining of apoptotic DNA damage using a TUNEL assay. (K) Effective transfection in HepG2 cells was confirmed using immunoblots. Values are represented as mean ± SD. *P < 0.05; †P < 0.05 vs. group 1; ††P < 0.05 vs. group 2. ANOVA was used for all statistical comparisons (A–J). Scale bars: J, 200 μm.