Abstract
A number of coronavirus disease 2019 (COVID-19) vaccine candidates have shown promising results, but substantial uncertainty remains regarding their effectiveness and global rollout. Boosting innate immunity with bacillus Calmette Guérin (BCG) or other live attenuated vaccines may also play a role in the fight against the COVID-19 pandemic. BCG has long been known for its nonspecific beneficial effects that are most likely explained by epigenetic and metabolic reprogramming of innate immune cells, termed trained immunity. In this issue of the JCI, Rivas et al. add to these arguments by showing that BCG-vaccinated health care providers from a Los Angeles health care organization had lower rates of COVID-19 diagnoses and seropositivity compared with unvaccinated individuals. Prospective clinical trials are thus warranted to explore the effects of BCG vaccination in COVID-19. We posit that beyond COVID-19, vaccines such as BCG that elicit trained immunity may mitigate the impact of emerging pathogens in future pandemics.
The time between SARS-CoV-2 emergence and an effective vaccine
Approximately one year ago, a new type of respiratory infection emerged in Wuhan, China. Shortly thereafter, the etiologic agent of this disease was described as a new coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), closely related to the agent of SARS, and the disease was termed coronavirus disease 2019 (COVID-19). The clinical spectrum of COVID-19 is heterogeneous, as approximately 40% of infected individuals are asymptomatic, a smaller percentage experience a mild upper respiratory tract infection, and a notable minority develop severe pneumonia with acute respiratory distress syndrome (ARDS), respiratory insufficiency, and even death (1). Although aggressive containment measures have been initiated by many countries, the pandemic could not be contained, with many countries in the Northern Hemisphere experiencing a second infection wave in the autumn-winter of 2020. It is expected that SARS-CoV-2 infection will become endemic, with regular outbreaks occurring either when quarantine measures are relaxed, or in the cold seasons in future years. Vaccination remains the most realistic hope to curb the spread of the virus. A number of vaccines have recently been reported to be effective against COVID-19, but it will take several months before a sufficient number of doses can be produced and distributed to the populations of the developed countries, and much longer to vaccinate the entire world’s population. In the time between the emergence of the virus and an effective worldwide vaccination, COVID-19 will have caused immense suffering and death for millions of people as well as disastrous economic consequences.
Because of the very high morbidity and mortality burden caused by COVID-19, alternative approaches for the prevention of the disease have been proposed. One approach is the repurposing of existing drugs such as hydroxychloroquine, lopinavir/ritonavir, remdesivir, or IFN-β, all of which have unfortunately shown disappointing effectiveness (2). Another interesting proposed strategy is to use the long-known, but mostly neglected, properties of some vaccines to induce cross-protection against infections outside the target disease (3). Vaccines that contain live attenuated microorganisms — in particular, the bacillus Calmette-Guérin (BCG) vaccine, measles-containing vaccines (such as measles, mumps, and rubella [MMR]), and the oral polio vaccine (OPV) — have such properties, as they improve mortality in children beyond the protection against their respective target diseases (4).
BCG-induced protection
BCG was developed against tuberculosis at the beginning of the 20th century at the Institut Pasteur in Paris, and since then it has been one of the most widely used vaccines in the world. After its introduction in various countries during the decades following its development, epidemiological surveys found that BCG vaccination strongly reduced infant mortality, an effect that could not be explained by a reduction in tuberculosis alone (reviewed in ref. 5). Interestingly, BCG-induced protection appeared to be due to effects against respiratory tract infections in particular (6), an observation also later confirmed in randomized trials in adults (7, 8). These clinical trials have been complemented by experimental studies that investigated the mechanisms through which BCG induces these protective effects. Spencer et al. showed that BCG vaccination reduced viral titers of influenza A virus in mice, an effect dependent on macrophages (9), suggesting strong effects on innate immunity. This hypothesis was strengthened by studies demonstrating increased monocyte function after BCG vaccination in human volunteers (10). Subsequently, it was discovered that the monocyte functional changes are accompanied by transcriptional, epigenetic, and metabolic reprogramming of the myeloid cell progenitors in BCG-vaccinated individuals (ref. 11 and Figure 1, A and B). The long-term changes in the innate immune cell phenotype after BCG vaccination amount to a de facto induction of innate immune memory, a phenomenon that was termed “trained immunity.” There is evidence that induction of trained immunity is, at least in part, the mechanism through which BCG vaccination provides its beneficial effects, also against viral infections (12). On the basis of these arguments, it was therefore hypothesized that BCG vaccination may be effective as a preventive measure against SARS-CoV-2 infection and may also reduce disease severity.
Figure 1. Model for trained immunity mechanisms that improve the antiviral host defense.
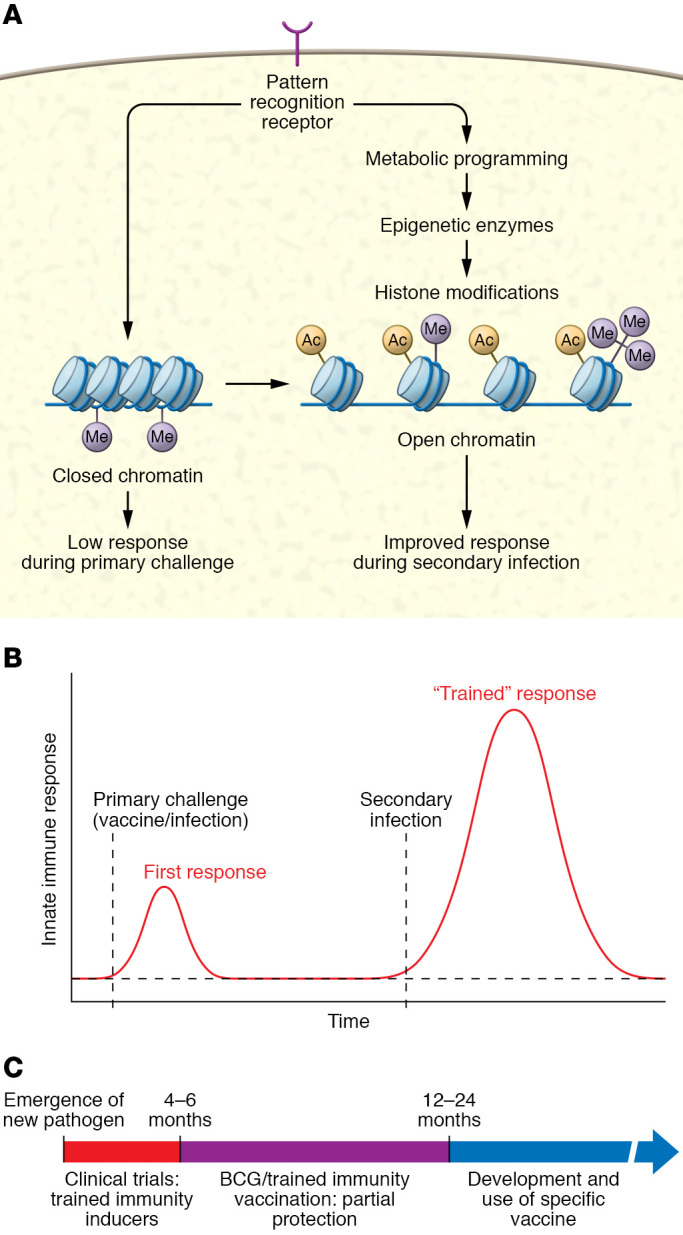
(A) Trained immunity is mediated by metabolic and epigenetic rewiring in the innate immune cells, leading to increased gene transcription and host defense against heterologous pathogens. (B) Following a first exposure to a live or live attenuated challenge, the innate immune response becomes poised via trained immunity to increase substantially following a second infection. (C) Trained immunity can be used as a tool for enhancing population immunity during a pandemic.
BCG–COVID-19 prevention hypothesis
This BCG–COVID-19 prevention hypothesis receives a boost by an elegant study published in this issue of the JCI by Rivas and colleagues (13). The authors assessed morbidity due to SARS-CoV-2 infections in a large cohort of health care professionals from a multisite Los Angeles health care organization. Almost one-third of the volunteers participating in the study had received an earlier BCG vaccination, which was accompanied by markedly fewer (30%–40%) self-reported COVID-19 diagnoses, self-reported positive COVID-19 reverse transcription PCR (RT-PCR) tests, and anti–SARS-CoV-2–seropositive tests. BCG vaccinations were shown to be associated with a lower incidence of COVID-19, despite the fact that the vaccinated group was slightly older and had more comorbidities. Symptoms associated with COVID-19 were also notably less severe in the BCG-vaccinated group than in the unvaccinated group. Interestingly, internal controls showed that such positive effects were not induced by other vaccinations such as those against influenza, meningococcus, or pneumococcus, so the effect seems to pertain to BCG immunization. It must be said, however, that the number of individuals who were not vaccinated against influenza was too small to draw strong conclusions (13).
These data are important, as they appear to support several epidemiological studies suggesting that countries with a BCG vaccination program have a lower number of COVID-19 infections and reduced mortality rates (13, 14, 15). However, demographic, ethnic, and genetic differences among populations in different countries, as well as differences in the social distancing measures and population compliance and in the diagnosis and reporting of COVID-19 cases all induce bias in such analyses, despite great efforts to correct for them. In addition, studies investigating vaccinated and nonvaccinated cohorts from countries that have stopped their BCG vaccination policy in recent years have found that the number of COVID-19 diagnoses did not differ, and hence early-life BCG vaccination is unlikely to provide protection (16). While these studies have provided important arguments cautioning against the conclusion that BCG vaccination at birth can protect against COVID-19, they do not reject the idea that BCG would be protective when given later in life, and especially shortly before the emergence of SARS-CoV-2. Although the study by Rivas et al. unfortunately did not collect data regarding the time of BCG vaccination, it is conceivable that the protective effect may have been due to more recent vaccinations. In line with recent vaccination protection findings, a recent observational survey of individuals vaccinated with BCG in the past two years (17) and one clinical study of health care personnel given BCG vaccination during the first phase of the pandemic (18) have both suggested protective effects of BCG against SARS-CoV-2 infection.
Conclusions and considerations
There is compelling support for BCG vaccination as a way to prevent COVID-19, and the study by Rivas and colleagues (13) provides an important piece of this puzzle. However, in order to be fully convinced that BCG vaccination can have such protective effects, randomized, controlled trials are needed that could provide the highest level of proof for the hypothesis that BCG vaccination protects against COVID-19. As more than 20 clinical trials are currently assessing the efficacy of BCG vaccination against COVID-19, we expect a clear answer soon.
Two RNA-based vaccines from BioNtech-Pfizer and Moderna were recently reported to confer more than 90% protection against COVID-19, including in the elderly. A third vaccine based on an adenovirus platform from Oxford-AstraZeneca also appears to provide at least 60% protection. Should BCG studies continue, given the high effectiveness of specific vaccines? There are several important arguments for why studies on the effects of the BCG vaccine (and other live attenuated vaccines) against COVID-19 are needed. First, it is yet unknown how long COVID-19–specific vaccines could provide protection, and one may hypothesize that a combination of BCG and a COVID-19–specific vaccine would induce lasting protection. Second, although it has been claimed that the new vaccines are protective in the elderly, we do not know the extent to which comorbidities, medication, and frailty will affect the vaccine response. Combining the COVID-19–specific vaccine with BCG may aid the specific immune response. Third, next year will be characterized by a shortage of the new COVID-19 vaccines, especially in developing countries. The availability of BCG, which does not necessitate storage at very low temperatures, could provide an important tool against the pandemic in areas where cooling facilities are in shortage. Finally, and maybe most important, it is crucial to learn whether live attenuated vaccines such as BCG protect against the spread of a severe infection during a pandemic: such a trained immunity–based vaccination may prove powerful in future pandemics against the uncontrolled spread of an emerging pathogen. BCG, and maybe other live attenuated vaccines, could represent a bridge vaccination during the period between the emergence of a new pathogen and the development of a specific vaccine, to diminish the suffering of the population from both the disease itself and its economic consequences (Figure 1C).
Acknowledgments
MGN was supported by a European Research Council (ERC) Advanced Grant (grant no. 833247) and a Spinoza Grant of the Netherlands Association for Scientific Research.
Version 1. 12/11/2020
In-Press Preview
Version 2. 01/19/2021
Print issue publication
Footnotes
Conflict of interest: MGN has patents (US18/61935, titled “Targeted nanoimmunotherapy to increase trained immunity” and US18/61939, titled “Targeted nanoimmunotherapy for inhibition of trained immunity”) and is a scientific founder of Trained Therapeutix and Discovery. He received unconditional research grants from ViiV HealthCare.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(2):e145545. https://doi.org/10.1172/JCI145545.
Contributor Information
Mihai G. Netea, Email: Mihai.Netea@radboudumc.nl.
Jos W.M. van der Meer, Email: jos.vandermeer@radboudumc.nl.
Reinout van Crevel, Email: reinout.vancrevel@radboudumc.nl.
References
- 1.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1056/NEJMoa2023184. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19 –interim WHO solidarity trial results. [published online December 2, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed]
- 3.Aaby P, et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol. 2020;20(8):464–470. doi: 10.1038/s41577-020-0338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins JP, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shann F. The non-specific effects of vaccines. Arch Dis Child. 2010;95(9):662–667. doi: 10.1136/adc.2009.157537. [DOI] [PubMed] [Google Scholar]
- 6.Stensballe LG, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23(10):1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Nemes E, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis EJ, et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183(2):315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer JC, et al. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with bacille Calmette-Guérin. J Infect Dis. 1977;136(2):171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- 10.Kleinnijenhuis J, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirovic B, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arts RJW, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Rivas MN, et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest. 2021;131(2):e145157. doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escobar LE, et al. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc Natl Acad Sci U S A. 2020;117(30):17720–17726. doi: 10.1073/pnas.2008410117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg MK, et al. Mandated bacillus Calmette-Guerin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci Adv. 2020;6(32):eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamiel U, et al. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323(22):2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorlag SJCFM, et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: a retrospective cohort study. Cell Rep Med. 2020;1(5):100073. doi: 10.1016/j.xcrm.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1101/2020.08.10.20172288. Amirlak I, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection [preprint]. Posted on medRxiv August 11, 2020. [DOI] [PMC free article] [PubMed]


