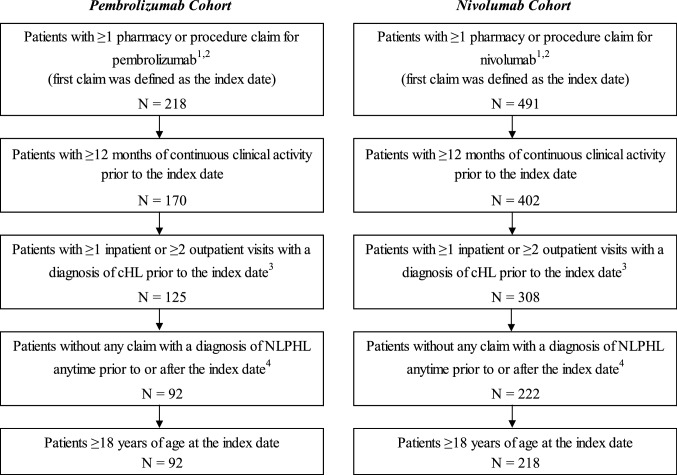
Fig. 1.
Sample selection. cHL classical Hodgkin lymphoma, GPI Generic Product Identifier, HCPCS Healthcare Common Procedure Coding System, ICD-9-CM International Classification of Diseases, 9th Revision, Clinical Modification, ICD-10-CM International Classification of Diseases, 10th Revision, Clinical Modification, NLPHL nodular lymphocyte-predominant Hodgkin lymphoma. 1 Patients who received both treatments and who initiated nivolumab prior to pembrolizumab approval or in the first months after were classified in the pembrolizumab cohort. Patients with an index treatment of pembrolizumab prior to nivolumab were considered as part of the pembrolizumab cohort (i.e., cohorts were mutually exclusive). 2 Pembrolizumab (GPI: 2135305300; HCPCS: J9271) and nivolumab (GPI: 2135304100; HCPCS: J9299) were identified from pharmacy and procedure claims. 3 Diagnosis of cHL in the primary or secondary position (ICD-9-CM: 201.0x–201.2x, 201.5x–201.9x; ICD-10-CM: C81.1x–C81.9x). 4 Diagnosis of NLPHL in the primary or secondary position (ICD-9-CM: 201.4x; ICD-10-CM: C81.0x)