Learning objectives.
By reading this article, you should be able to:
-
•
Counsel, before surgery, older patients at risk of postoperative cognitive dysfunction (POCD).
-
•
Refer patients for neuropsychometric testing to assess preoperative cognitive function.
-
•
Make adjustments to perioperative care and anaesthesia to reduce the risk of POCD in high-risk individuals.
Key points.
-
•
Postoperative cognitive dysfunction (POCD) is a possible, subtle decline in cognition present after a patient has recovered from the acute impact of surgery and hospital stay.
-
•
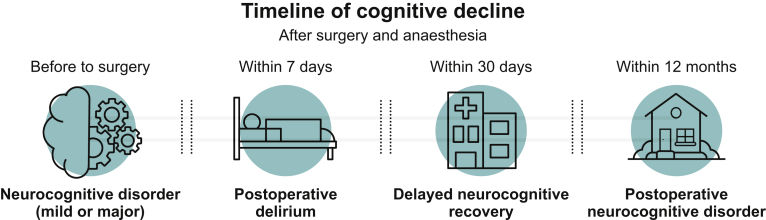
Postoperative cognitive dysfunction is newly defined as ‘delayed neurocognitive recovery’ if present within 30 days of surgery, or ‘postoperative neurocognitive disorder’ if present within 1 yr after surgery.
-
•
The inconsistent diagnosis and testing of POCD have led to significant variation between published studies.
-
•
There is moderate evidence to suggest that altering some aspects of anaesthesia may reduce the risk of developing POCD.
Advanced age in surgical patients is accompanied by life-altering and costly complications. This challenge is keenly felt in high-income countries, where the mean age for elective surgery is 58 yrs, nearly 10 yrs older than in low-income countries.1 In the UK, one in five individuals are aged >65 yrs. A national enquiry into patient outcomes in 2010, ‘Knowing the Risk’, identified that nearly 80% of postoperative deaths were in the high-risk group of patients; these deaths occurred in only 10% of the total numbers undergoing surgery. Advanced age, alongside comorbidities, such as disability and frailty that often develop with age, are all independent markers of such risk.2 A common factor in this group is cognitive impairment, which can be a great challenge to navigate during the perioperative period for both the patient and clinicians. It affects consent and optimisation before surgery; existing diseases may progress in severity during the perioperative period, and new symptoms of delirium or postoperative cognitive dysfunction (POCD) can complicate postoperative recovery.
The relationship between cognition and surgery has been recognised for a long time. The British Medical Journal first published work in 1887 entitled, ‘Insanity following the use of anæsthetics in operations’, which described ‘a series of cases of insanity in which the use of anaesthetics, in predisposed subjects, has been followed by insanity’ (Fig. 1).3 This was a milestone in the field and represents an early effort to understand the involvement at different degrees of anaesthesia and surgery.
Fig 1.
First publication about POCD was published by George H. Savage in 1887. Reproduced from Savage, p. 1190, with permission from BMJ Publishing Group Ltd.3
Ancient historical accounts of delirium, derived from the Latin term meaning ‘off the track’, date back to Hippocrates in 400 BC (Aphorisms, section VII).4 Hippocrates considered delirium to be a consequence of disease, often following fever, dyspnoea or meningitis. He pointed out how psychomotor hyperactivity, an impaired sleep/wake pattern, inability to recognise known people and difficulty in completing easy tasks were the most common characteristics of people affected and could be often considered as fatal signs (Aphorisms, section IV).
Our current understanding of postoperative delirium is well informed, as delirium is often encountered within clinical practice. The development of POCD, a decline in cognitive function from the patient's baseline, is less well identified. This is frequently a subtle change, but leaves patients less able to live independently at their expected quality of life. As described by the mantra of The King's Fund, ‘no decision about me, without me’,5 it is highly important to offer informed counsel on the potential impact of surgery and anaesthesia on a patient's medium-to long-term cognition.
In this article, we outline current research on preventative measures that reduce the burden of surgery and anaesthesia on cognitive function. Despite cognitive syndromes being noted many centuries ago, there is much inconsistency surrounding POCD in the literature. Research methodology has varied, with inconsistent diagnostic criteria, and the progress made can easily remain unnoticed.
Postoperative neurocognitive disorders
Cognitive impairment after surgery and anaesthesia may present as a progression of existent neurocognitive disorder (NCD), acute onset of delirium or development of POCD. The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) recently amended its diagnostic criteria, which may be reported by the patient or an informant, or tested by a clinician:6
-
(i)
Mild NCD: noticeable decline in cognitive function, requiring adjustment to maintain independence in activities of daily living that extends beyond the normal changes of ageing
-
(ii)
Major NCD: significant burden of cognitive impairment that results in impaired activities of daily living
The reduced functional ability differentiates between major and mild NCDs, which, as a spectrum of impairment, may be challenging to distinguish. Dementia may describe further aetiological subtypes (e.g. Alzheimer's disease, vascular dementia and substance-induced dementia). The DSM-5 outlines the following cognitive domains evaluated in NCD:
-
(i)
Learning and memory: ability to learn and recall new information
-
(ii)
Language: comprehension or expression
-
(iii)
Perceptual motor: visual perception and coordination
-
(iv)
Social cognition: insight and recognition of emotions
-
(v)
Complex attention: sustained, divided or selective attention and speed of processing
-
(vi)
Executive function: planning, decision-making and flexibility
-
(vii)
Delirium: an acutely fluctuating disturbance in attention, awareness and cognition developed over a short period of time
Postoperative cognitive dysfunction is not yet defined by the DSM-5. It is described in the literature as a postoperative decline in cognitive function that can potentially last from months to years.
Delirium
The fluctuating changes in attention, level of consciousness and cognitive function in delirium occur acutely within hours or days. It is widely considered to be triggered by surgery and anaesthesia when presenting 24–72 h postoperatively in the absence of alternative aetiologies, although not specified by the DSM-5. It is highly prevalent in surgical patients, and can occur in 15–53% of older patients after surgery and 70–87% of those in critical care.6 Delirium can manifest in a hypo- or hyperactive state. Hyperactive delirium is easily detected clinically, with features of psychomotor agitation, reduced inhibitions and restlessness that may threaten the patient's own safety. The incidence of hypoactive delirium is likely underestimated, as the patient may appear calm, but upon examination shows signs of disorientation, inattention and reduced mobility.
Postoperative cognitive dysfunction
Postoperative cognitive dysfunction describes a decline in cognitive ability from a patient's baseline that starts in the days after surgery and is prevalent in 1% of elderly patients after 1 yr.7 Cognitive decline may be noted across one or multiple of the cognitive domains. For the patient, this may result in difficulty writing, managing money or remembering lists, and can have a very tangible effect on their lives after discharge home. A study of patients with hip fractures concluded those who developed POCD had poorer ability to function socially and manage activities of daily living at 1 yr.8 A Danish study of 700 patients followed-up for 8 yrs found that early POCD (at 1 week) was associated with individuals leaving the labour market prematurely and withdrawing social benefit payments.9 Furthermore, POCD carries an increased risk of death 1 yr after surgery.9,10 Follow-up more than 11 yrs after surgery has shown no association between POCD and dementia.11
The time frame of onset of POCD remains undefined, but it can be detectable from 7 days after surgery.12 Transient cognitive deficits immediately after surgery have multifactorial causes (emergence from anaesthesia, sleep deprivation, pain, anxiety, polypharmacy, inadequate nutrition and operative complications), and these causes, alongside postoperative delirium, will significantly undermine neuropsychological testing for POCD. It is our belief that changes in cognition earlier than 7 days after surgery cannot be accurately tested and attributed to POCD.
The incidence of POCD in elderly patients at 1 week is 30%, at 3 months is 10–13% and at 1 yr is 1%.10,13,14 These data describe non-cardiac surgical patients, as the neurobehavioural consequences of cardiac surgery are well established and documented, with cardiopulmonary bypass likely to independently contribute to cognitive decline.15,16 Whilst POCD can affect patients of any age, it has more profound sequelae in the elderly. A handful of well-designed studies have compared the prevalence of POCD with age-matched non-surgical controls, and report a 3% incidence of POCD in the general population at 1 week and 3 months.14 Another study has shown cognitive decline in older subjects to occur, similar to POCD, irrespective of surgery or major illness.15 Although the authors accept some limitations to the work, their findings question the existence of POCD as an entity. We would highlight that their conclusions are founded on age-matched controls, where the surgical groups studied for POCD are highly likely to be comorbid, as evidenced by the need for surgery. Bias may be introduced here, as age-matched controls do not represent similarly comorbid patients. It is a challenge to implement the research needed for clarity on the phenomenon of POCD, and only when large prospective trials match the surgical group to disease-matched controls will there be a weight of evidence that can deny or confirm the existence of such cognitive disease.
Further controversy surrounds the clinical tools used to diagnose POCD. To best detect subtle and specific neurocognitive changes, diagnosis should include a battery of tests that span the cognitive domains described previously. The most current research has studied POCD through assessment with the simple Abbreviated Mental Test or Mini-Mental State Examination (MMSE). These brief and accessible tests were designed for dementia screening and are too crude to identify subtle features of cognitive decline. There are multiple options for sensitive neuropsychometric testing, such as the Montreal Cognitive Assessment tool, Addenbrooke's cognitive examination III and the Quick Mild Cognitive Impairment screen.12
Montreal Cognitive Assessment is an accessible 10 min test, evaluating each of the cognitive domains and has been validated to screen for mild cognitive impairment (MCI), which, by the DSM-5 definition, requires a new onset of deficit in at least two areas of cognitive functioning.6 Elements of the test will be familiar to clinicians, such as the clock drawing task that is often used in frailty assessment and high-risk preoperative clinics. This test offers promise for global consensus in assessment of POCD, having been widely translated for global use and modified for cultural and linguistic differences. Validation of this, or another, tool for POCD requires analysis in a large RCT, with disease-matched controls.
Single-point testing is inadequate to detect POCD, as diagnosis requires a preoperative baseline and postoperative assessment to quantify a decline in cognition. Consensus is required to agree the degree of decline accepted as diagnostic for POCD; herein lies another key element impairing the current quality of evidence in the literature. Proposed statistical methods include the following:
-
(i)
Percentage change method: (postoperative score–preoperative score)/preoperative score
-
(ii)
Z-score: the number of sd the observed measurement of decline is from the mean of a control group. The control group mean is determined by testing a control group twice at the same time interval as the preoperative and postoperative testing in the intervention (surgical) group; Z-score=(observation–mean)/sd. A Z-score of 2 sd from the mean is considered diagnostic of POCD.12
Neuropsychometric testing needs to become embedded in the holistic perioperative care of the elderly surgical population. This was highlighted by the 2015 Association of Anaesthetists' guideline, ‘Perioperative care of people with dementia’, which stated the need for preoperative assessment processes that identify patients with baseline cognitive impairment. Such testing would be costly, and the optimal timing remains undetermined, but it is key to better recognition of this perioperative complication.
Proposals for new definitions
As yet, POCD is not a formal psychiatric diagnosis, and the wide variation in clinical diagnosis of POCD is to the detriment of patient care and ongoing research. This has been addressed by the International Perioperative Cognition Nomenclature Working Group through discussion amongst an expert panel, which recently proposed a new narrative for describing perioperative cognitive disorders.17 It aims to align the diagnostic criteria of POCD with NCDs defined by the DSM-5 and already used in the general (non-surgical) population. Figure 2 outlines the suggested definitions.
Fig 2.
Proposed definitions of perioperative NCDs.
The newly named ‘postoperative neurocognitive disorder’ (postoperative NCD) is ideally measured by a battery of neuropsychometric tests. A reduction of 1–2 sd defines postoperative mild NCD, and >2 sd defines postoperative major NCD. Where assessment of change from baseline is not possible, norms or control groups should be used. The working group does not recommend which psychometric tests to perform or which cognitive domains to interrogate. It directs clinicians away from early testing for POCD (<7 days), emphasising the need for the acute effects of surgery and hospitalisation to have resolved before a cognitive impairment diagnosis. Appropriate assessment of multiple cognitive domains should be the basis for diagnosis, with serial assessment to increase accuracy of diagnosis.
The efforts are valuable, and it is the responsibility of the wider medical community to embrace change that advances consistency in the diagnosis of POCD. Remaining gaps can be considered opportunity for further research to validate neuropsychometric tests and cement progress made.
Aetiology of POCD
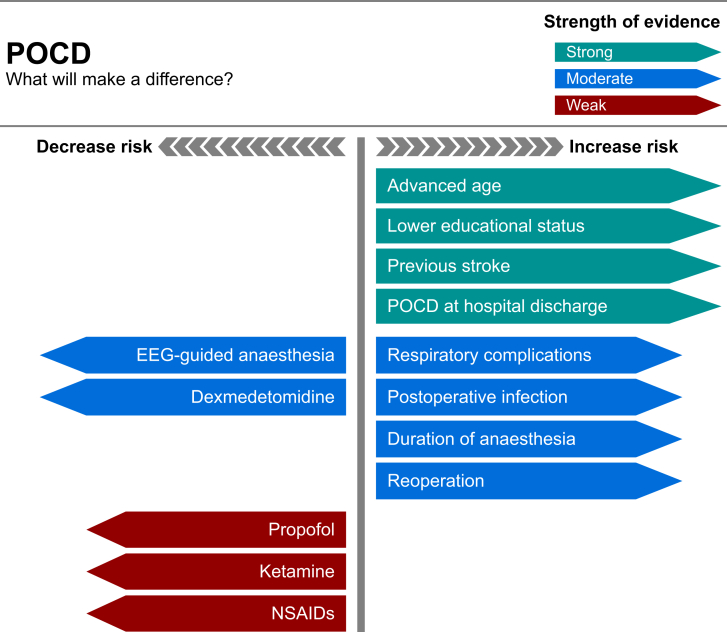
The risk factors identified for POCD are advancing age, lower educational level and a history of a previous cerebral vascular accident with no residual impairment. Both preoperative MCI and cognitive dysfunction at hospital discharge correlate with POCD at 3 months after surgery.8,10,18 Further studies have suggested predictors for early POCD to include the duration of anaesthesia, postoperative infections, second operations and respiratory complications.14
The pathogenesis of POCD remains unclear, with research focusing upon the role of neuronal death, neuroinflammation and micro-emboli. Animal in vivo studies have shown that inhalational anaesthetic agents potentiate neuronal death through the degradation of the cholinergic system by amyloid plaques and neurofibrillary tangles.10,12 These cholinergic pathways are a key element of consciousness, learning and memory. Conversely, there has been no relationship established between POCD and apolipoprotein E genotype carriers, a strong risk factor for Alzheimer's disease, which is characterised by loss of basal forebrain cholinergic neurones.19 Neuroinflammation occurs as inhalational agents increase the permeability of the endothelial cells in the cerebral vasculature, allowing cytokines to enter and damage neural tissue.20 Micro-emboli from the surgical site or air entrainment may cause cerebral infarcts, and have been studied using MRI, but no clear relationship was found (i.e. to pre-existing conditions, such as patent foramen ovale).12
Neuroimaging has been studied to discern brain alterations relating to POCD. A systematic review found weak evidence to suggest that POCD correlates with reduced thalamic volume, reduced hippocampal volume, pre-existing white matter pathology and reduced cerebral blood flow.16
Prevention
Key to the management of NCDs is prevention through optimised perioperative care (Fig. 3). How can the anaesthetist's management influence the patient's outcomes?
Fig 3.
Infographic detailing strength of evidence for factors that increase and decrease the risk of POCD.
General or regional anaesthesia
Current understanding of the pathophysiology of POCD is thought to be cholinergic dysfunction in response to neuroinflammation and synaptic impairment. Many, therefore, believe regional anaesthesia may limit development of NCDs. However, current research has failed to identify such a relationship.
A 2018 systematic review of 1,031 patients across seven studies, considering the impact of regional vs general anaesthesia on POCD suggested a higher incidence of POCD during the first 3 days postoperatively with general anaesthesia, but no difference from Day 7 postoperatively.21 They included brief comment on the neuropsychometric assessment, ‘MMSE or neuropsychological tests were used’, suggesting conclusions were drawn without a consistent measurement of cognitive decline. Furthermore, the authors describe POCD as manifesting as insanity and anxiety, symptoms indicative of delirium.
A larger meta-analysis (2,365 patients) found no difference in the incidence of POCD after regional or general anaesthesia.22 The analysis recognised the variance in testing and categorised neurological deterioration between cortical functions (e.g. decline in memory, concentration and visual and spatial skills) for separate interrogation, demonstrating one method of drawing together conclusions from research that utilises varying measurements of cognitive decline.
Inhalational or i.v. maintenance of anaesthesia
A 2018 Cochrane review found low-certainty evidence that propofol-based TIVA may reduce POCD (odds ratio 0.52; 95% confidence interval [CI] 0.31–0.87; 869 participants).23 Starting from 3,215 eligible patients, the analysis was hampered by vast heterogeneity in methodology, emphasising the need for consistency in POCD definition.
This follows smaller RCTs, which have compared propofol and sevoflurane and found a correlation with sevoflurane increasing the incidence of POCD at 7 days and 9 months postoperatively.20,24
This effect may be linked to EEG-guided anaesthesia, often coupled with propofol for maintenance anaesthesia, into which there is a considerable amount of research.
EEG-guided or clinical monitoring of depth of anaesthesia
Recent evidence has highlighted that titrating general anaesthesia through the use of bispectral index (BIS) monitoring or auditory-evoked potential monitoring reduces cognitive dysfunction at 3 months postoperatively.25 The Cognitive Dysfunction after Anesthesia trial indicated that 23 patients per 1,000 were prevented from developing POCD at 3 months, by implementing BIS-guided anaesthesia (adjusting anaesthetic dosage to achieve a BIS of 40–60).26 The impact on POCD at 1 week and 1 yr remains uncertain. Conversely, a meta-analysis of four studies showed no correlation between depth of anaesthesia and POCD.27 These conclusions are inconsistent, suggesting there is a benefit from the use of cerebral monitoring during anaesthesia, which is independent of the cerebral monitoring values.
Studies vary in methodological rigour and cognitive testing, so it is with caution that we suggest BIS-guided anaesthesia, which is titrated to avoid excessive depth of anaesthesia or awareness, may reduce POCD.
Clinical variables
No association has been found between abnormal physiology, such as hypoxia, hypotension or altered cerebral perfusion, and increased risk of POCD.12 A large multicentre trial studied pulse oximetry and arterial pressure as markers of cerebral oxygenation, and did not find any correlation with POCD.14
Dexmedetomidine
Dexmedetomidine is recognised to have neuroprotective effects and is considered to reduce delirium perioperatively. As a central alpha-2 adrenoreceptor agonist, it acts at the locus coeruleus to cause sedation, opioid-sparing analgesia and cardiac depression, and imitates physiological-type sleep without impacting respiratory function. Possible mechanisms for the ability of dexmedetomidine to reduce POCD include inhibition of inflammation, analgesia and CNS protection. In a meta-analysis of seven studies that used dexmedetomidine within general anaesthesia, POCD at Day 7 postoperatively was significantly lower in the treatment group (R=0.34; 95% CI 0.19–0.61; p<0.001).28 The RCTs studied only assessed cognition with postoperative MMSEs and no baseline assessment, which weakens the certainty of this association.
Ketamine
Ketamine is an N-methyl-D-aspartate antagonist, with anaesthetic and analgesic properties, recognised to exhibit neuroprotective effects. A systematic review that compared giving ketamine during surgery with no intervention found some evidence of protection from POCD with ketamine, but the quality of the evidence was very low.29 The majority of studies included a single i.v. bolus of ketamine. However, the effects of a single bolus of ketamine are short lived and using an infusion would better suit studies of its potential neuroprotective effects.
Steroids
Postoperative cognitive dysfunction has been shown to correlate with high concentrations of inflammatory markers (C-reactive protein [CRP] and interleukin-6 [IL-6]) in the peripheral circulation and CSF, which can be targeted by glucocorticoids.30 However, a meta-analysis found no impact of dexamethasone on the incidence of POCD at 30 days postoperatively, founded on limited, varied data from three studies.31 In addition, the presence of systemic IL-6 and CRP may be an indicator of a larger physiological response that would not be lessened with steroids. We suggest that cytokine-mediated inflammation is likely not the sole pathogenesis of POCD, and consider the altered glucose metabolism caused by steroids to pose potential risk to neurocognition.
Non-steroidal anti-inflammatory drugs
The anti-inflammatory agent parecoxib, a selective cyclooxygenase-2 inhibitor, has been shown in preclinical studies to reduce levels of the neurotoxic inflammatory mediators S100 calcium-binding protein B (S100B) and neurone-specific enolase, which clinically correlate with POCD.32 A meta-analysis, pooling four RCTs of 900 patients, demonstrated that parecoxib was effective at reducing the incidence of POCD at 7 days postoperatively, alongside reduced levels of S100B and IL-6.33 This supports the theory that S100B production is involved in POCD pathogenesis, but the strength of evidence is limited by three of these RCTs using MMSE for neurocognitive assessment.
Conclusions
As POCD becomes a clearer-, better-defined clinical entity, it mandates transparent discussion with patients during consent for surgery and anaesthesia. One in 10 elderly patients will suffer with POCD at 3 months postoperatively, and for some, this reduced quality of life outweighs the benefits of surgery.
Until there is routine preoperative neuropsychometric assessment of patients over 65 yrs, there is an absence of appropriate preoperative counselling or recognition of cognitive decline post-intervention. The implementation of testing and consent is best placed in preoperative clinics, a valuable opportunity to discuss cognitive decline after surgery with both the patients and their families, who will often play a significant role in recognising cognitive decline and advocating for the patients.
Recent advances in the study of POCD include the working group offering a quantified measure of decline for diagnosis of POCD, alongside a new narrative. They emphasise the importance of avoiding premature testing, which confounds results with the acute sequelae of surgery and anaesthesia. There remains a lack of validated neuropsychological testing for POCD, which is required for homogeneous research methodology. We recommend that future research should use a neuropsychometric assessment tool that measures each of the six cognitive domains and defines POCD quantitatively with a Z-score calculation.
Tools at the anaesthetist's disposal that show promise to reduce the incidence of POCD include EEG-guided anaesthesia, propofol TIVA and dexmedetomidine, with further study of ketamine needed.
With a commitment to appropriate testing and consent that leads to robust research methodology, there will be increasing strength of evidence to confirm which adjustments to anaesthetic management do indeed give the patient the best quality of life after surgery.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Mario Cibelli MD MSc PhD MRCP FRCA FFICM is a consultant in anaesthesia and intensive care at the University Hospitals Birmingham, and senior lecturer at the University of Birmingham, and his PhD was in neurosciences. His other interests include delirium, postoperative cognitive dysfunction (POCD), pain and regional anaesthesia. He is a lead contributor to the Royal College of Anaesthetists' information programme for patients on delirium and POCD.
Elizabeth Brodier BMedSci FRCA is a specialty registrar in anaesthesia at the Birmingham School, West Midlands Deanery. She has an interest in research and collaborates with several research projects. Dr Brodier has recently secured an NIHR grant as a collaborator in a project on fast-track cardiac surgery.
Matrix codes: 1F01, 1H02, 1I05, 2A03, 3I00, 3J02
References
- 1.International Surgical Outcomes Study group Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Confidential Enquiry into Patient Outcome and Death . 2010. An age old problem. A review of the care received by elderly patients undergoing surgery. London. [Google Scholar]
- 3.Savage G.H. Insanity following the use of anæsthetics in operations. Br Med J. 1887;2:1199–1200. [Google Scholar]
- 4.Aphorisms by Hippocrates, written 400 B.C. Available from: classics.mit.edu/Hippocrates/aphorisms.html. [Accessed 7 December 2020].
- 5.Coulter A., Collins A. Making shared decision-making a reality: No decision about me, without me. www.kingsfund.org.uk 2011. Available from: [Accessed 7 December 2020]
- 6.American Psychiatric Association . 5th Edn. American Psychiatric Association; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 7.Abildstrom H., Rasmussen L.S., Rentowl P. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. ISPOCD group. International Study of Post-Operative Cognitive Dysfunction. Acta Anaesthesiol Scand. 2000;44:1246–1251. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 8.Gruber-Baldini A.L., Zimmerman S., Morrison R.S. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51:1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz J., Christensen K.B., Lund T., Lohse N., Rasmussen L.S. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 10.Monk T.G., Price C.C. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17:376–381. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmetz J., Siersma V., Kessing L.V., Rasmussen L.S. Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. Br J Anaesth. 2013;110:92–97. doi: 10.1093/bja/aes466. [DOI] [PubMed] [Google Scholar]
- 12.Needham M.J., Webb C.E., Bryden D.C. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119:115–125. doi: 10.1093/bja/aex354. [DOI] [PubMed] [Google Scholar]
- 13.Paredes S., Cortínez L., Contreras V., Silbert B. Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2016;60:1043–1058. doi: 10.1111/aas.12724. [DOI] [PubMed] [Google Scholar]
- 14.Moller J.T., Cluitmans P., Rasmussen L.S. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 15.Avidan M.S., Searleman A.C., Storandt M. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Mårtensson J., Gögenur I., Asghar M.S. Exploring postoperative cognitive dysfunction and delirium in noncardiac surgery using MRI: a systematic review. Neural Plast. 2018;2018:1281657. doi: 10.1155/2018/1281657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evered L., Silbert B., Knopman D.S. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. Br J Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silbert B., Evered L., Scott D.A. Preexisting cognitive impairment is associated with postoperative cognitive dysfunction after hip joint replacement surgery. Anesthesiology. 2015;122:1224–1234. doi: 10.1097/ALN.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 19.Cao L., Wang K., Gu T., Du B., Song J. Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta-analysis. Int J Neurosci. 2014;124:478–485. doi: 10.3109/00207454.2013.860601. [DOI] [PubMed] [Google Scholar]
- 20.Qiao Y., Feng H., Zhao T., Yan H., Zhang H., Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 2015;15:154. doi: 10.1186/s12871-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Liu D., Wang B., Li X., Zhang S. Effects of general anesthesia and non-general anesthesia on postoperative cognitive dysfunction in patients: a systematic review. Int J Clin Exp Med. 2018;11:12253–12258. [Google Scholar]
- 22.Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: a meta-analysis. Indian J Anaesth. 2011;55:358–363. doi: 10.4103/0019-5049.84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller D., Lewis S.R., Pritchard M.W. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018;8:CD012317. doi: 10.1002/14651858.CD012317.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micha G., Tzimas P., Zalonis J., Kotsis K., Papadopoulos G., Arnaoutoglou E. Propofol vs sevoflurane anaesthesia on postoperative cognitive dysfunction in the elderly. A randomized controlled trial. Acta Anaesth Belg. 2016;67:129–137. [PubMed] [Google Scholar]
- 25.Punjasawadwong Y., Chau-In W., Laopaiboon M., Punjasawadwong S., Pin-On P. Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non-cardiac and non-neurosurgical procedures in adults. Cochrane Database Syst Rev. 2018;5:CD011283. doi: 10.1002/14651858.CD011283.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan M.T.V., Cheng B.C.P., Lee T.M.C., Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 27.Lu X., Jin X., Yang S., Xia Y. The correlation of the depth of anesthesia and postoperative cognitive impairment: a meta-analysis based on randomized controlled trials. J Clin Anesth. 2018;45:55–59. doi: 10.1016/j.jclinane.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang W., Kong L.S., Zhu X.X., Wang R.X., Liu Y., Chen L.R. Effect of dexmedetomidine on postoperative cognitive dysfunction and inflammation in patients after general anaesthesia: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2019;98:e15383. doi: 10.1097/MD.0000000000015383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hovaguimian F., Tschopp C., Beck-Schimmer B., Puhan M. Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2018;62:1182–1193. doi: 10.1111/aas.13168. [DOI] [PubMed] [Google Scholar]
- 30.Liu X., Yu Y., Zhu S. Inflammatory markers in postoperative delirium (POD) and cognitive dysfunction (POCD): a meta-analysis of observational studies. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L.-Q., Wang C., Fang M.-D., Xu H.-Y., Lu H.-L., Zhang H.-Z. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. 2019;19:113. doi: 10.1186/s12871-019-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X., Wen L.J., Cui C., Li D.R., Teng J.F. The significance of S100β protein on postoperative cognitive dysfunction in patients who underwent single valve replacement surgery under general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:2192–2198. [PubMed] [Google Scholar]
- 33.Huang S., Hu H., Cai Y.H., Hua F. Effect of parecoxib in the treatment of postoperative cognitive dysfunction: a systematic review and meta-analysis. Medicine. 2019;98 doi: 10.1097/MD.0000000000013812. [DOI] [PMC free article] [PubMed] [Google Scholar]