Figure 1.
Wild-type p53 forms stable cytoplasmic aggregates that reduce p53 activity
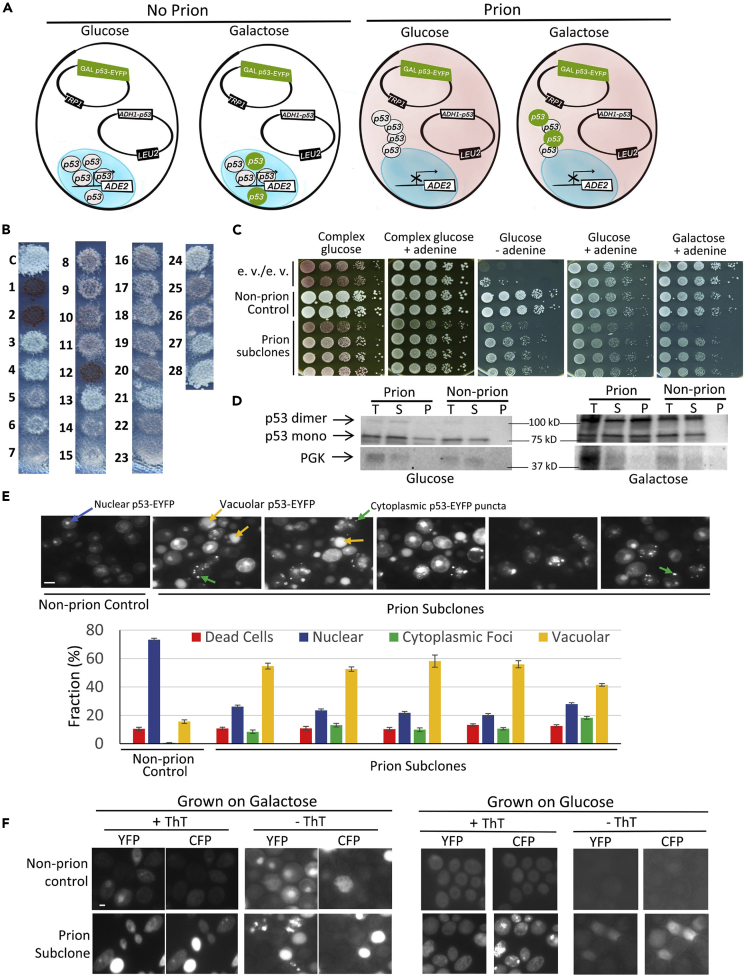
(A) Cartoon of strain L3671 in which prion candidates were obtained showing cells with and without prion grown on glucose versus galactose. Shown are plasmids pADH1-p53 and pGAL1-p53-EYFP and ADE2 reporter gene under p53 control, contained in L3671. The nucleus is depicted as a blue oval. Even though the plasmids are nuclear, they are shown in the cytoplasm to make it easier to see them. In the absence of prion, p53 is in the nucleus where it is diffuse and turns on the ADE2 gene, making cells white and able to grow in the absence of adenine. In the presence of prion, p53 forms cytoplasmic puncta, reducing the amount of p53 in the nucleus so that ADE2 is not transcribed efficiently, and the cells are reddish in color on most media and grow poorly in the absence of adenine. L3671 grown on glucose only expresses untagged p53, from the constitutive ADH1 promoter. L3671 grown on galactose also expresses p53 tagged with EYFP from the GAL1 promoter. The nuclei in cells without the prion grown on galactose (but not glucose) show diffuse green fluorescence. Likewise, the cytoplasmic p53 foci in prion cells are only fluorescently tagged when grown on galactose.
(B) p53 prion candidates have reduced p53 activity. Cells shown are on plasmid-selective, adenine-limiting, glucose medium. The L3671 control (C) and prion candidates (1–28) are shown. Reduced expression of ADE2, and therefore reduced p53 activity, is seen as a pinkish color and reduced growth on media with limited adenine. Expression of p53 cannot be estimated in candidates 1, 2, or 12, because they are [rho−] and this loss of mitochondria caused them to become dark red.
(C) Reduced ADE2 expression is stably maintained in a p53 prion candidate. Following extensive culturing, normalized suspensions of subclones of prion candidate #7 (L3672), non-prion control (L3671) and empty vector (e.v.) control strain (yIG397), were one-tenth serially diluted in water and spotted on YPD (complex glucose) with and without additional adenine, on plasmid-selective synthetic glucose (glucose) with and without adenine, and on plasmid-selective 2% galactose medium with adenine plates. These plates were photographed after 3–5 days of incubation at 30°C. Red papillations on the non-plasmid-selective YPD plate without excess adenine result from loss of the p53 plasmid.
(D) p53 sediments more in lysates of prion candidates than in non-prion controls. Non-prion control (L3671) and prion (L3672) cells grown in plasmid-selective glucose or galactose media were lysed as described in Transparent Methods. Total lysates (T) were separated into Supernatant (S) and Pellet (P) by centrifugation at 80K RPM for 30 min. Samples were run on SDS-PAGE, and gels were immunoblotted with anti-p53, stripped, and reprobed with anti-PGK for a loading control. Detection of p53-EYFP monomer (~70 kD) or dimer and PGK (44.7 kD) on western blot was based on migration of Precision Plus Protein Dual Color (Bio Red, Hercules, CA).
(E) Prion candidate has p53-EYFP cytoplasmic foci. Both non-prion control (L3671) and prion candidate (L3672) repeatedly subcloned cells, grown on galactose to induce expression of GAL1-p53-EYFP, were examined under a fluorescent microscope and photographed (top). The fraction of cells with nuclear fluorescence (Nuclear), cytoplasmic aggregates (Cytoplasmic Foci), or vacuolar fluorescence (Vacuolar) was determined, counted, and converted into % fraction (bottom). Trypan blue was used to stain dead cells. Standard error of the mean bars shown were based on nine replicates and 200–700 cells were scored for each replica. The prion and non-prion cultures were statistically different (p < 0.05%) for fractions of cells with nuclear fluorescence, cytoplasmic puncta, and vacuolar fluorescence.
(F) p53 prion-like aggregates stain with Thioflavin T. Fluorescence of non-prion control (L3671) and prion cells (L3672) both containing pADH1-p53 and pGAL1-p53-EYFP grown on plasmid-selective galactose (left) or glucose (right) plates, fixed and stained (+ThT) or not stained (- ThT) with Thioflavin T versus EYFP fluorescence were, respectively, detected under CFP versus YFP filters. Cells expressing EYFP that were stained with Thioflavin T exhibited reduced, but detectable, EYFP fluorescence. Scale bar, 5 μM. Note: dead cells often look bright due to increased autofluorescence.
See Figures S1 and S2 for additional characterization of p53 non-prion and prion strains and Tables S1 and S2 for a description of strains and plasmids.