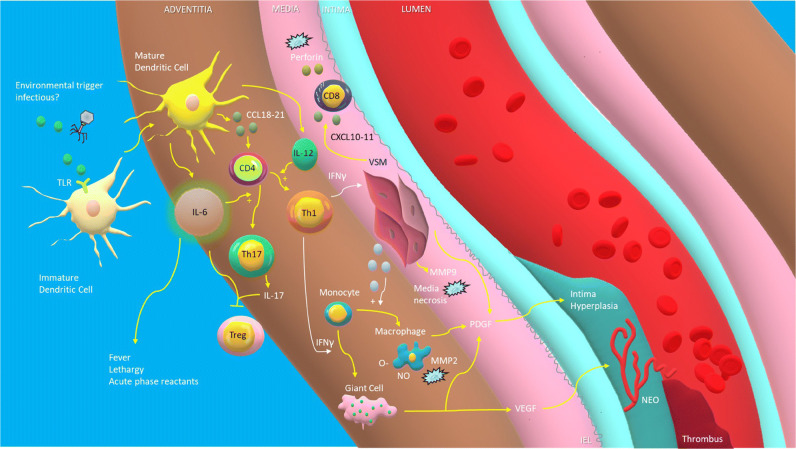
Fig. 1.
Pathogenesis of giant cell arteritis. Unknown environmental stimuli, possibly infectious, activate immature dendritic cells within the adventitia of blood vessels through stimulation of receptors such as the toll like receptor (TLR), leading to the release of chemokines (CCL18–21) that recruit naive CD4+ helper T cells. These T cells, under the influence of interleukin-6 (IL-6), differentiate into Th17 cells which produce IL-17, while others, triggered by IL-12, differentiate into Th1 cells which release interferon gamma (INF-γ). Il-6 and IL-17 may at the same time lead to a reduction in regulatory T cells (Treg). INF-γ causes vascular smooth muscle cells (VSM) to release cytokines that recruit monocytes which are transformed into either macrophages or multinucleated giant cells under the influence of INF-γ. Macrophages release reactive oxygen species that peroxides phospholipids in cellular membranes and matrix metalloproteinase-2 (MMP-2) which, along with MMP-9 released by VSM, destroy cellular matrix proteins such as elastin, resulting in the destruction of the media. CXCL10–11 released by VSM cells recruit CD8+ T cells which release cytotoxic perforin. Macrophages, injured VSM, and giant cells all release platelet-derived growth factor (PDGF) which leads to intimal hyperplasia and associated luminal stenosis, which in turn can lead to luminal thrombosis. Vascular endothelial growth factor (VEGF) released by giant cells leads to neoangiogenesis. TLR, troll-like receptor. CD4, undifferentiated CD4+ helper T cell. INF-γ, interferon gamma. VSM, vascular smooth muscle cells. O-, reactive oxygen species. NO, nitric oxide. Treg, regulatory T cell. MMP, matrix metalloproteinase. CD8, CD8+ killer T cells. PDGF, platelet-derived growth factor. VEGF, vascular endothelial growth factor. NEO, neoangiogenesis. IEL, internal elastic lamina.