Fig. 4. ICOS expression on activated T cells in the human PBMCs-NSG GVHD model correlates with disease severity, and the suppressive effects of ALPN-101 are not altered by CsA.
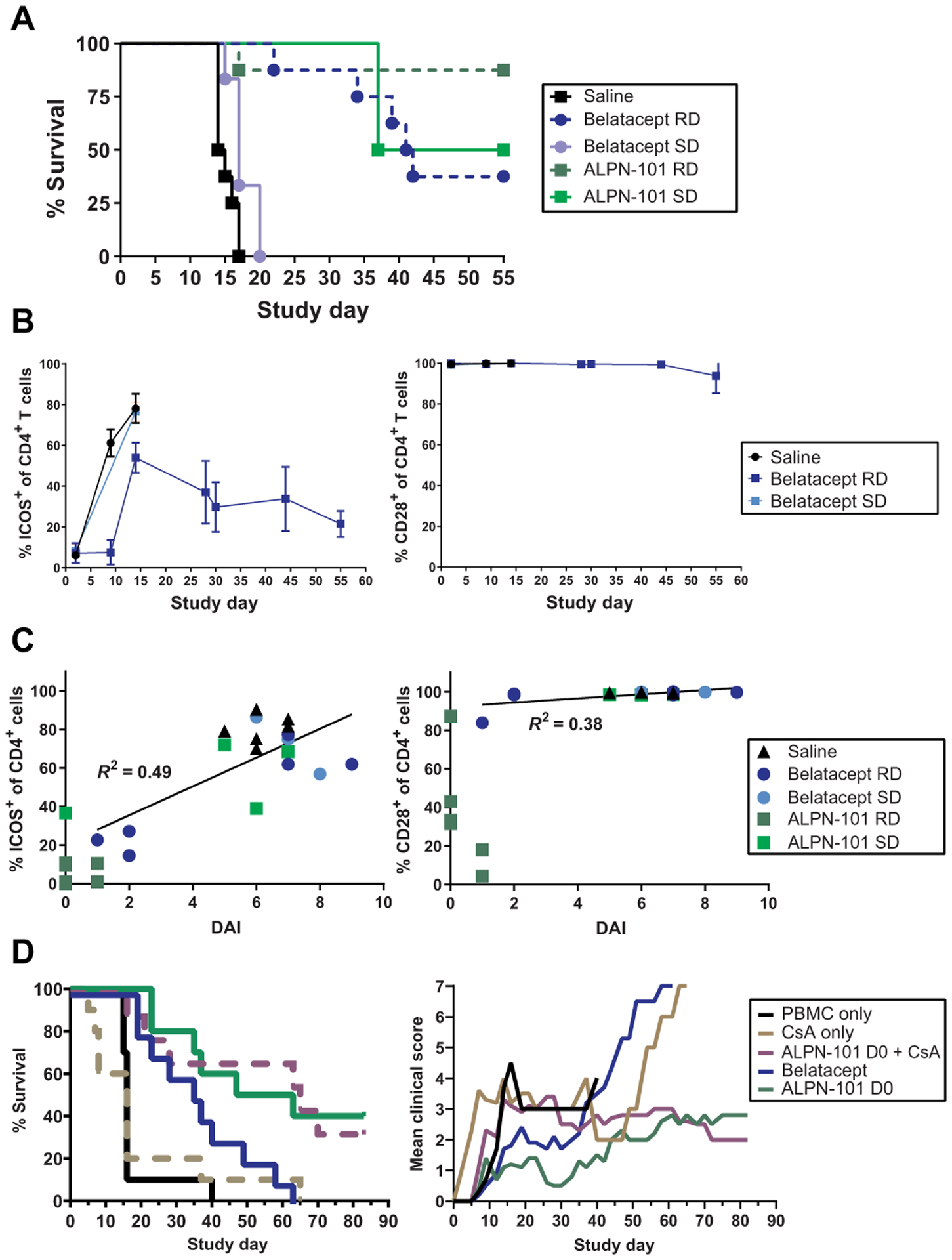
(A) Survival of mice receiving repeated doses (RD) or a single dose (SD) of the indicated test articles. NSG mice x-ray irradiated (100 cGy) and administered 10 mg of human γ globulin subcutaneously on day −1 and then transplanted intravenously with 10 × 106 human PBMCs on day 0 were treated intraperitoneally with saline; 100 μg of ALPN-101 TIW × 4 (days 0 to 28) or once on day 0; or 100 μg of belatacept TIW × 4 (days 0 to 28) or once on day 0. (B) Blood from each mouse treated with saline or a single dose (SD) or repeat doses (RDs) of belatacept or ALPN-101 [as described in (A)] was evaluated by flow cytometry for percent cells positive for ICOS or CD28 in the population of human CD4+ cells. Because ALPN-101 blocks the binding of anti-ICOS and anti-CD28 antibodies used for flow cytometry, data from the ALPN-101 groups are omitted. (C) Percent ICOS+ or CD28+ human CD4+ cells are plotted versus the terminal DAI score for each mouse as described in (A). Linear regression curves were calculated and correlation coefficients (R2) are indicated for each dataset. Because ALPN-101 blocks the binding of the antibodies used for flow cytometry, data from the ALPN-101 groups are shown on the graphs but were omitted from the correlation analyses. See table S7 for additional correlation analyses. (D) Effect of CsA on ALPN-101 therapy. Percent survival and clinical scores for NSG mice x-ray irradiated (100 cGy) and administered 10 mg of human γ globulin SC on day −1, then transplanted intravenously with 10 × 106 human PBMCs on day 0 (D0) and treated intraperitoneally with saline daily from day −1 to 13, then TIW through day 28; CsA 20 mg/kg daily from day −1 to 13, then TIW through day 28; 500 μg of ALPN-101 once on day 0; combination of 500 μg of ALPN-101 once on day 0 plus CsA 20 mg/kg daily from day −1 to 13, then TIW through day 28; or 75 μg of belatacept TIW × 4. In (A) (left), statistical significance in survival proportions between groups was determined by Mantel-Cox log-rank test. In (B), data are shown as mean ± SEM. In (D) (left), statistical significance in survival proportions between groups was determined by Mantel-Cox and Gehan-Breslow-Wilcoxon log-rank tests. In (D) (right), statistical significance was determined by two-way repeated measures ANOVA for treatment effect.
