Figure 1.
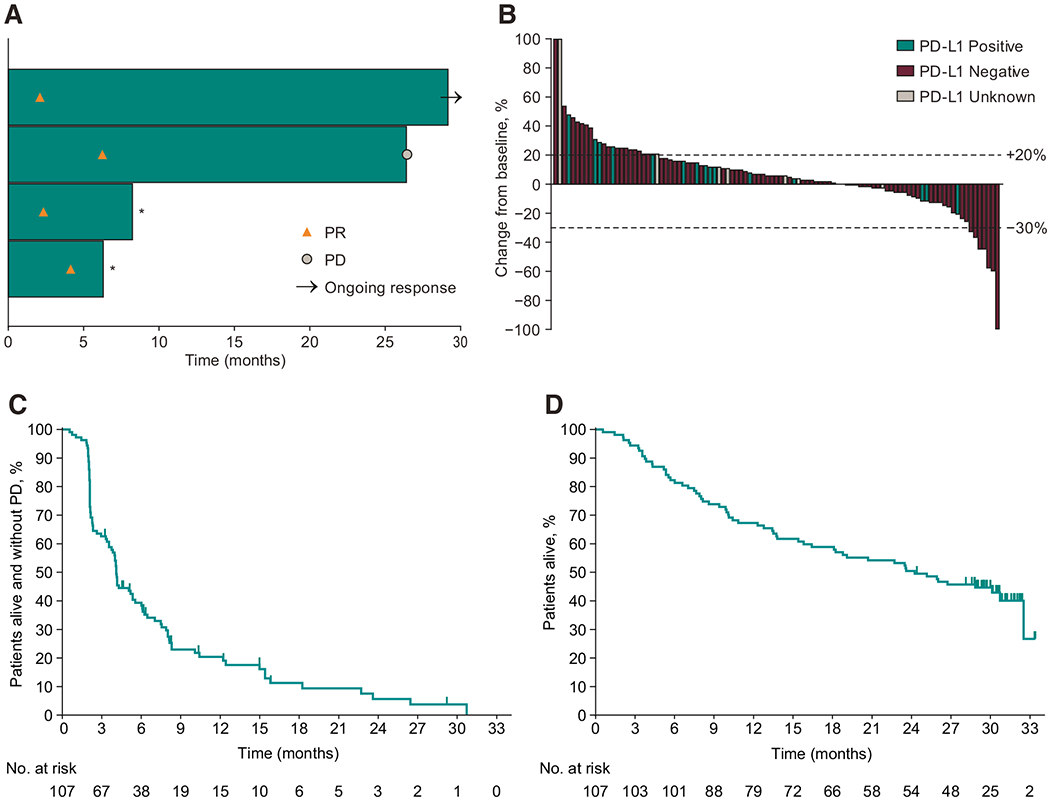
Antitumor activity of pembrolizumab in the total population. A, Time to and duration of response assessed by RECIST v1.1 per independent central review in patients whose best overall response was partial response (n = 4). The length of the bars represents the time to the last imaging assessment. *Started new anticancer therapy without progressive disease. PR, partial response. B, Best change from baseline in target lesion size assessed by RECIST v1.1 per independent central review in patients with ≥1 evaluable postbaseline imaging assessment (n = 101). C, Kaplan-Meier estimates of progression-free survival assessed by RECIST v1.1 per independent central review in the efficacy population (N = 107); D, Kaplan-Meier estimates of overall survival in the efficacy population (N = 107). PR, partial response.
