Abstract
Objective: ACE2 is crucially involved in the infection sustained by SARS-CoV-2, as it allows the entry of the virus into target cells while counteracting local inflammation, oxidative stress, and fibrosis. In this narrative review, we aim to discuss the usefulness of ACE2-derived peptides in the infection sustained by SARS-CoV-2. Methods: A total of 49 papers pertinent to the purpose of the review were selected from the PubMed and Google Scholar databases. Clinical trials registered at ClinicalTrials.gov and dealing with the use of ACE2-derived medications in COVID-19 were also searched and discussed. Results: Preclinical and clinical evidence shows that drugs mimicking or potentiating the effects of ACE2 may reduce the viral load and dampen the inflammatory and fibrotic pathways leading to respiratory distress. ACE2-derived therapeutic peptides may have a better pharmacokinetic and pharmacodynamic profile than other ACE2-based medications. They could be easily screened through peptide libraries and chemically modified in order to ameliorate the pharmacological properties. Furthermore, their local administration via an intranasal delivery or inhalation may reduce the risk of systemic side effects, thus conferring a good safety profile. Conclusion: ACE2-derived peptides may play a dual beneficial role in COVID-19, by either preventing virus spread or inhibiting the secretion of pro-inflammatory mediators in airways. Viral, host, and environmental factors may affect the effectiveness of this therapeutic approach to a various extent and represent therefore a matter of investigation for future studies.
Keywords: SARS-CoV-2, COVID-19, angiotensin-converting enzyme 2, therapeutic peptides
Introduction
The renin-angiotensin system (RAS) plays a crucial role in the infection sustained by severe acute respiratory syndrome (SARS)-coronavirus (CoV)-2 and may dictate the following course of COronaVIrus Disease 2019 (COVID-19). This disease is, in fact, extremely variable in terms of severity and type of clinical manifestations, leading, in worst cases, to an acute respiratory distress syndrome (ARDS) and death [1]. SARS-CoV-2 can be transmitted via either the aerial or the fecal-oral route [2]. Specifically, the viral spike (S) protein may bind the angiotensin-converting enzyme 2 (ACE2), localized on the apical membrane of epithelial and endothelial cells of lungs and small intestine [3,4], and, in synergy with the host’s transmembrane serine protease 2 (TMPRSS2), allow the membrane fusion promoted by envelope viral proteins [5]. ACE2 is a membrane carboxypeptidase homolog to ACE (dipeptidyl carboxypeptidase), from which it differs in the target-binding domain [6]. ACE converts the decapeptide angiotensin I (Ang I) into the octapeptide angiotensin II (Ang II) [7,8], and is mainly responsible for the control of blood pressure [9]. ACE2, instead, converts Ang II into angiotensin-(1-7) (Ang-[1-7]) and counterbalances ACE activity [10]. Besides the endocrine cardiovascular effects, the RAS acts through a paracrine mechanism in almost all the human tissues [11]. In airways, Ang II may bind angiotensin receptor 1 (AT1) and lead to the production of reactive oxygen species (ROS) and collagen deposition by lung fibroblasts [12]. Conversely, Ang-(1-7) and ACE2 seem to have a protective effect and prevent the activity of ACE in a negative feedback loop [13]. By converting Ang II into Ang-(1-7), the enzyme ACE2 may, in fact, reverse vasoconstriction and impart anti-oxidative and anti-inflammatory signals to lung cells [14]. Ang II signaling via AT2 was shown to have similar effects by contrasting the pro-inflammatory cascade promoted by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [15]. Experiments conducted on animal models showed that lung ACE2 prevents hypo-oxygenation, alveolar edema, inflammatory cell recruitment, and hyaline membrane formation following lung acid aspiration or H5N1 virus infection [16,17]. Consequently, the balance between ACE and ACE2 in alveoli is likely to be critically important in the control of inflammation developing in response to an external agent. By binding ACE2 receptors in the lungs, SARS-CoV-2 may switch off this feedback mechanism and drive the inflammatory process [18,19].
The crucial role played by ACE2 in the development of COVID-19 is also supported by epidemiological and genetic data. Specifically, the hyperexpression of ACE2 in testis, heart, and adipose tissue [20-22] may explain some demographical and clinical differences reported in SARS-CoV-2-infected patient cohorts, like the occurrence of the most severe forms of COVID-19 in male, obese and cardiopathic individuals [23-25].
Moreover, polymorphic variants of the ACE2 gene have been associated with diabetes mellitus, hypertension, coronaropathy, and left ventricular hypertrophy [26-28] that represent altogether risk factors for a severe prognosis of COVID-19 [1], and may be at the basis of different rates of morbidity and mortality observed in Caucasian and Chinese populations [29].
Based on these data, it is quite probable that targeting the local RAS in airways would be a promising therapeutic strategy for COVID-19 [9].
Specifically, drugs mimicking the biological effects of ACE2 may impede SARS-CoV-2 infection while in the meantime rebalancing local inflammation.
Thanks to their efficacy and safety profile, ACE2-derived peptides may particularly stand out in the category of RAS-targeting medications. Therapeutic peptides consist of short amino acid chains able to selectively interact with receptors or other molecules of interest [30]. Modifications in their molecular structure may ameliorate their pharmacological profile and even confer multiple ligand affinities [30], thus strengthening the pharmacological effects. Finally, local administration into airways may result in less harmful side effects than systemic treatments.
The aim of this review is to discuss preclinical and clinical evidence supporting the plausible usefulness of ACE2-derived medications in COVID-19 patients, with a special focus on ACE2-derived peptides, compared to other drugs targeting the RAS.
Methods
The PubMed and Google Scholar databases were searched using the combination of words “SARS-CoV-2”; “renin-angiotensin system”; “ACE2”; “ACE2-derived peptides”; “COVID-19”. A total of 49 papers published from 1997 to date, written in English and pertinent to the aim of the review, were selected and discussed. Additionally, clinical trials registered at ClinicalTrials.gov and dealing with the use of ACE2-derived medications in COVID-19 were searched and reviewed. Results are reported below.
Results
ACE-inhibitors
The effectiveness of ACE-inhibitors in treating or preventing COVID-19 is unclear [1,31]. These drugs were reported to selectively prevent the action of ACE without affecting that of ACE2 [32]. When administered to animal models together with AT1 antagonists, ACE-inhibitors could induce the over-expression of ACE2. In turn, the ACE2 increase may have both favorable (anti-inflammatory) and unfavorable (host cell infection) repercussions on the COVID-19 course. However, recent data did not show evidence of worse COVID-19 outcomes in infected individuals who took these medications [33-35].
Angiotensin-(1-7)
Ang II is cleaved by endopeptidases and mono-carboxypeptidases, including ACE2, to generate the heptapeptide Ang-(1-7). By binding G-coupled protein Mas receptors, Ang-(1-7) counteracts arterial hypertension, aging, inflammation, oxidative stress, and cell proliferation [14]. Most of these effects are due to the antagonistic role played by Ang-(1-7) on Ang IIrelated pathways. In airways, Ang-(1-7) protects alveolar cells from apoptosis, reduces inflammation and fibrosis, and impedes the activation of endothelial cells [36]. Based on this evidence, two clinical trials (ClinicalTrials.gov ID NCT04375124 and NCT04332666) have been designed in order to investigate the beneficial role of this heptapeptide for the treatment of COVID-19 patients.
Recombinant human ACE2 and ACE2 homologs
Another therapeutic approach would be the use of recombinant human (rh)ACE2 or ACE2 homologs in SARS-CoV-2-infected individuals, which may prevent both viral dissemination and inflammation [37]. Given at an appropriate concentration in order to saturate ligand binding, these drugs might neutralize the S protein of SARS-CoV-2, which will eventually impede the entry of the virus into epithelial and endothelial cells, without affecting the physiologic ACE/ACE2 balance.
The beneficial effect played by rhACE2 in reducing respiratory distress and inflammation and constraining viral load has been shown in preclinical studies on ARDS, SARS, and H5N1 influenza animal models [16,17,19]. On the basis of these encouraging data, rhACE2 was developed and tested in ARDS patients in a phase II randomized controlled trial [38]. Although no improvements in the clinical outcomes were reported, the parenteral administration of the drug induced a higher conversion of Ang II into Ang-(1-7) and Ang-(1-5) isoforms and a decreased production of pro-inflammatory cytokines, like interleukin-6 (IL-6). Notably, the treatment with agents antagonizing the IL-6 receptor has shown encouraging results in some patients affected by COVID-19 [39]. Another study in animal models reported that rhACE2 may constrain pulmonary arterial hypertension by increasing the expression of antioxidant enzymes like superoxide dismutase, with no effect on bleomycin-induced lung fibrosis [40]. Soluble rhACE2 has been recently tested in vitro in Vero-E6 cells, human vascular cells and kidney organoids exposed to SARS-CoV-2: notably, the compound significantly reduced virus entry into target cells, appearing therefore to be useful during the early stages of COVID-19 [41]. Unfortunately, a small open-label pilot study (ClinicalTrial.gov ID NCT04287686), aiming to enroll at least 24 COVID-19 patients to be assigned to an intravenous treatment with rhACE2 (given at a dose of 0.4 mg/kg twice a day for 7 days), was recently withdrawn. Therefore, the clinical efficacy of rhACE2 in COVID-19 remains unclear.
Bacteria can represent a further source of naturally synthesized ACE2-like enzymes. Paenibacillus sp. B38 produces a carboxypeptidase (B38-CAP) able to convert Ang II into Ang-(1-7). The administration of B38-CAP to animal models was shown to revert Ang II-related arterial hypertension, and to prevent cardiac hypertrophy and fibrosis with negligible immunogenicity rates [42]. Accordingly, two phase I randomized clinical trials are currently evaluating this therapeutic strategy in COVID-19 patients.
Finally, immune cells can be engineered in order to express ACE2 on their surface while delivering potent antiviral cytokines: an ongoing phase I/II randomized controlled clinical trial is evaluating the therapeutic effect of ACE2- and superagonist-IL-15-expressing natural killer (NK) cells in severe COVID-19 pneumonia.
ACE2-derived peptides
Besides rhACE2, ACE2-derived therapeutic peptides, containing the crucial amino acids interacting with the S1 subunit of the SARS-CoV-2 S protein, could represent an alternative strategy to target this pathway. ACE2-derived peptides could be easily screened through peptide libraries and chemically modified in order to potentiate their pharmacological properties [43].
Therapeutic peptides usually display a less harmful safety profile than other pharmaceutical compounds as they are rapidly biodegraded [30], and may be locally delivered to affected organs. In this regard, ACE2-derived peptides could be administered via an intranasal route or inhalation. This would allow them to directly exert their therapeutic effect in airway mucosa, without systemically affecting the RAS, Figure 1 [44,45]. Additionally, they could be opportunely modified in order to mimic those ACE2 variants which are less permissive to virus engagement [46].
Figure 1.
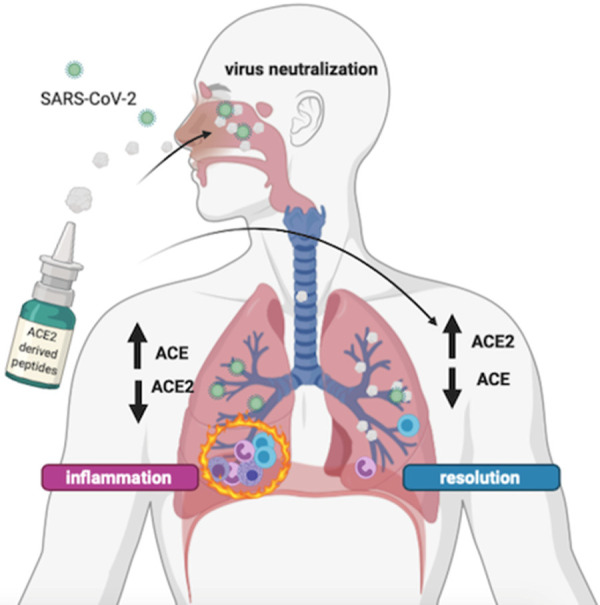
Plausible beneficial mechanism of human ACE2-derived peptides in preventing and treating SARS-CoV-2 infection. ACE2-derived peptides, containing the crucial amino acid residues that interact with the S protein of SARS-CoV-2, may be locally administered via an intranasal delivery or inhalation. These drugs may prevent virus entry into target cells and play an anti-inflammatory, anti-oxidative, anti-fibrotic, and anti-apoptotic role in epithelial and endothelial cells by antagonizing the effects of ACE. Abbreviations: ACE, angiotensin-converting enzyme; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Pharmacologic research is already moving in this direction. Specifically, computational analysis studies yielded a series of ACE2-derived peptides potentially binding and neutralizing the receptor-binding domain (RBD) of the SARS-CoV-2 S protein. It was shown that crucial amino acid residues placed at the 21-57 and 351-357 positions of the N-terminal helix of ACE2 allow the interaction with SARS-CoV-2 RBD [45,47]. By using automated fast-flow peptide synthesis, Zhang et al. synthesized a 23-mer fully human peptide derived from the α1 helix of ACE2 and binding the RBD with low nanomolar affinity [48]. Redesigned peptides may have better pharmacodynamic properties than native peptides: by linking the 22-44 and 351-357 amino acid residues with glycine and introducing strategic amino acid substitutions, Huang et al. synthesized a 31-mer peptide scaffold with enhanced binding affinity for SARS-CoV-2 RBD [43]. Lupala et al. designed nine ACE2-derived peptides (12-70 amino acids placed in the N-terminal helix), which were tested in molecular dynamics simulations. The authors reported that the amino acid residues 24-26 and 39-42 are crucial for RBD binding and that longer peptides have higher stability in water solution, which is maintained after target binding [47].
Clinical studies aiming to investigate the therapeutic role of ACE2-mimicking agents in COVID-19 patients are resumed in Table 1.
Table 1.
Summary of the clinical trials investigating the efficacy and safety of ACE-2 mimicking drugs in COVID-19
| Trial ID | Experimental intervention | Rationale for the use of this therapeutic strategy in COVID-19 | Phase | Design | Primary Endpoint | Estimated participants | Status |
|---|---|---|---|---|---|---|---|
| NCT04375046 | B38-CAP (bacteria-derived ACE2-like enzyme) 0.4 mg/kg intravenously given twice a day for 7 days in addition to standard of care | Preclinical evidence | I | Randomized, open-label, parallel assignment, controlled clinical study vs. standard of care (no PBO) | 1) Time course of body temperature (14 days) | 24 | Not yet recruiting |
| - antagonistic effect of B38-CAP on Ang II-induced arterial hypertension, cardiac hypertrophy and fibrosis in murine models | 2) Viral load over time (14 days) | ||||||
| NCT04382950 | B38-CAP (bacteria-derived ACE2-like enzyme) 0.4 mg/kg intravenously given twice a day for 7 days plus inhaled aerosolized 13 cis-retinoic acid for 14 days | I | Randomized, interventional, open-label, parallel assignment, controlled clinical study vs. standard of care (no PBO) | 1) Time course of body temperature in 14 days | 24 | Not yet recruiting | |
| NCT04324996 | NK cells | Preclinical evidence | I/II | Randomized, interventional, quadruple, parallel assignment, controlled study | 1) Efficacy of NKG2D-ACE2 CAR-NK cells in treating severe and critical COVID-19 pneumonia | 90 | Recruiting |
| IL15-NK cells | - potentiation of the antiviral response through the generation of engineered NK cells expressing ACE2 | 2) Side effects in the treatment group (time frame: up to 28 days) | |||||
| NKG2D CAR-NK cells | |||||||
| ACE2 CAR-NK cells | |||||||
| NKG2D-ACE2 CAR-NK cells (all the treatments intravenously given at a dose of 10E8 cells per kg of body weight once a week) | |||||||
| NCT04287686 | 0.4 mg/kg rhACE2 intravenously administered twice a day for up to 7 days in addition to standard of care | Preclinical evidence | N.A. | Open-label, randomized, 2-arm paralleled assignment, controlled, pilot clinical study | 1) Time course of body temperature (14 days) | 24 | Withdrawn (not approved by the Chinese Center for Drug Evaluation) |
| - prevention of the development of pulmonary arterial hypertension in mouse models and increased expression of SOD | 2) Viral load over time (14 days) | ||||||
| -reduced entry of SARS-COV-2 in Vero-E6 cells, human vascular cells and kidney organoids preincubated in vitro with rhACE2 | |||||||
| - increased survival rate, decreased pulmonary viral load and serum levels of Ang II in H5N1 influenza mouse models treated with rhACE2 compared to untreated animals | |||||||
| - amelioration of lung acute injury (edema and elastance) following acid aspiration in Ace2 knockout mice intraperitoneally injected with rhACE2 compared with controls | |||||||
| Clinical studies | |||||||
| - reduction of IL-6 serum levels and increase in surfactant protein D concentrations in ARDS patients parentally treated with rhACE2; no improvement in clinical outcomes |
Abbreviations: ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; PBO, placebo; B38-CAP, Paenibacillus sp. B38 carboxypeptidase; NK, natural killer; IL15, interleukin-15; CAR, chimeric antigen receptor; COVID-19, coronavirus disease 19; rh, recombinant human; SOD, superoxide dismutase; IL-6, interleukin-6; ARDS, acute respiratory distress syndrome.
Conclusion
ACE2-derived peptides, directly delivered in airways, may play a dual beneficial role in preventing the entry of the virus into target cells and in inhibiting the local secretion of pro-inflammatory mediators.
Although this pharmacologic scenario appears promising and rapidly evolving, several points should however be considered: 1) ACE2-derived peptides could be useful only in the first phase of the infection whilst the efficacy in established disease is uncertain; 2) the influence of demographic and environmental factors (age, comorbidities, other respiratory infections, cigarette smoking, and air pollution) on the effectiveness of this pharmacologic intervention in lungs should be delineated; 3) genomic instability of SARS-CoV-2 may favor the occurrence of variants in the S protein gene, which may result in the generation of protein isoforms with different affinity and avidity for ACE2-derived peptides; 4) as SARS-CoV-2 infection may spread, in some cases, through the gastrointestinal tract, the formulation of orally administered peptidase-resistant drugs, like peptidomimetic small molecules [49], should also be evaluated.
Disclosure of conflict of interest
None.
References
- 1.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) - StatPearls - NCBI Bookshelf. Treasure Island (FL): Edited by StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 2.Nikhat S, Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci Total Environ. 2020;728:138859. doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Baker A. Recombinant human ACE2: Acing out angiotensin II in ARDS therapy. Crit Care. 2017;21:305. doi: 10.1186/s13054-017-1882-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Ulitzky L, Silberstein E, Taylor DR, Viscidi R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26:126–132. doi: 10.1089/vim.2012.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy JL, Jackson RM, Acharya KR, Sturrock ED, Hooper NM, Turner AJ. Angiotensin-Converting Enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence. Biochemistry. 2003;42:13185–13192. doi: 10.1021/bi035268s. [DOI] [PubMed] [Google Scholar]
- 7.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177:4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77:301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 11.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 12.Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX, Li W, Li Y, Zhang WY, Li X. The angiotensin-converting enzyme 2/angiotensin (1-7)/mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxidants Redox Signal. 2015;22:241–258. doi: 10.1089/ars.2013.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783–792. doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 14.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1-7)-Mas receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 2014;4:201. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L, Carretero OA, Xu J, Harding P, Ramadurai N, Gu X, Peterson E, Yang XP. Activation of angiotensin II type 2 receptor suppresses TNF-α-induced ICAM-1 via NF-κb: possible role of ACE2. Am J Physiol - Hear Circ Physiol. 2015;309:H827–H834. doi: 10.1152/ajpheart.00814.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, Guo F, Bai T, Han Z, Zhu J, Zhou H, Huang F, Li C, Lu H, Li N, Li D, Jin N, Penninger JM, Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocrine, Metab Immune Disord - Drug Targets. 2020;20:807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 19.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo H, Wang X, Chen C, Wang J, Li C, Xu Z, Yang X, Shi W, Zeng C. Oxidative stress causes imbalance of renal renin angiotensin system (RAS) components and hypertension in obese Zucker rats. J Am Heart Assoc. 2015;4:e001559. doi: 10.1161/JAHA.114.001559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, Lew RA. The novel Angiotensin-Converting Enzyme (ACE) homolog, ACE2, is selectively expressed by adult leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 23.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34:339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 24.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Liu WY, George J, Zheng MH. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu YH, Li JY, Wang C, Zhang LM, Qiao H. The ACE2 G8790A polymorphism: involvement in type 2 diabetes mellitus combined with cerebral stroke. J Clin Lab Anal. 2017;31:e22033. doi: 10.1002/jcla.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu N, Yang Y, Wang Y, Liu Y, Fu G, Chen D, Dai H, Fan X, Hui R, Zheng Y. ACE2 gene polymorphism and essential hypertension: an updated meta-analysis involving 11,051 subjects. Mol Biol Rep. 2012;39:6581–6589. doi: 10.1007/s11033-012-1487-1. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Huang W, Su S, Li B, Zhao W, Chen S, Gu D. Association study of ACE2 (angiotensin I-converting enzyme 2) gene polymorphisms with coronary heart disease and myocardial infarction in a Chinese Han population. Clin Sci. 2006;111:333340. doi: 10.1042/CS20060020. [DOI] [PubMed] [Google Scholar]
- 29.Delanghe JR, Speeckaert MM, De Buyzere ML. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr Clin Res Rev. 2020;14:283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 33.de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O, Centeno-Soto GA, Ángeles Gálvez M, Puerro M, González-Rojano E, Pedraza L, de Pablo I, Abad-Santos F, Rodríguez-Mañas L, Gil M, Tobías A, Rodríguez-Miguel A, Rodríguez-Puyol D MED-ACE2-COVID19 study group. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peiró C, Moncada S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation. 2020;141:1665–1666. doi: 10.1161/CIRCULATIONAHA.120.047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 38.Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, Hardes K, Powley WM, Wright TJ, Siederer SK, Fairman DA, Lipson DA, Bayliffe AI, Lazaar AL. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan F, Fabbri L, Stewart I, Robinson K, Smyth AR, Jenkins G. A systematic review of Anakinra, Tocilizumab, Sarilumab and Siltuximab for coronavirus-related infections. medRxiv. 2020;2020:1–35. [Google Scholar]
- 40.Rathinasabapathy A, Bryant AJ, Suzuki T, Moore C, Shay S, Gladson S, West JD, Carrier EJ. rhACE2 therapy modifies bleomycin-induced pulmonary hypertension via rescue of vascular remodeling. Front Physiol. 2018;9:271. doi: 10.3389/fphys.2018.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minato T, Nirasawa S, Sato T, Yamaguchi T, Hoshizaki M, Inagaki T, Nakahara K, Yoshihashi T, Ozawa R, Yokota S, Natsui M, Koyota S, Yoshiya T, Yoshizawa-Kumagaye K, Motoyama S, Gotoh T, Nakaoka Y, Penninger JM, Watanabe H, Imai Y, Takahashi S, Kuba K. B38-CAP is a bacteria-derived ACE2-like enzyme that suppresses hypertension and cardiac dysfunction. Nat Commun. 2020;11:1058. doi: 10.1038/s41467-020-14867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X, Pearce R, Zhang Y. Computational design of peptides to block binding of the SARS-CoV-2 spike protein to human ACE2. bioRxiv. 2020:2020. doi: 10.18632/aging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fellner RC, Terryah ST, Tarran R. Inhaled protein/peptide-based therapies for respiratory disease. Mol Cell Pediatr. 2016;3:16. doi: 10.1186/s40348-016-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han Y, Král P. Computational design of ACE2-based peptide inhibitors of SARS-CoV-2. ACS Nano. 2020;14:5143–5147. doi: 10.1021/acsnano.0c02857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han DP, Penn-Nicholson A, Cho MW. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lupala CS, Kumar V, Li X, Su X, Liu H. Computational analysis on the ACE2-derived peptides for neutralizing the ACE2 binding to the spike protein of SARS-CoV-2. bioRxiv. 2020;2020:1–27. [Google Scholar]
- 48.Zhang G, Pomplun S, Loftis AR, Loas A, Pentelute BL. The first-in-class peptide binder to the SARS-CoV-2 spike protein. bioRxiv. 2020;2020:1–15. [Google Scholar]
- 49.Pauletti GM, Gangwar S, Siahaan TJ, Aubé J, Borchardt RT. Improvement of oral peptide bioavailability: peptidomimetics and prodrug strategies. Adv Drug Deliv Rev. 1997;27:235–256. doi: 10.1016/s0169-409x(97)00045-8. [DOI] [PubMed] [Google Scholar]


