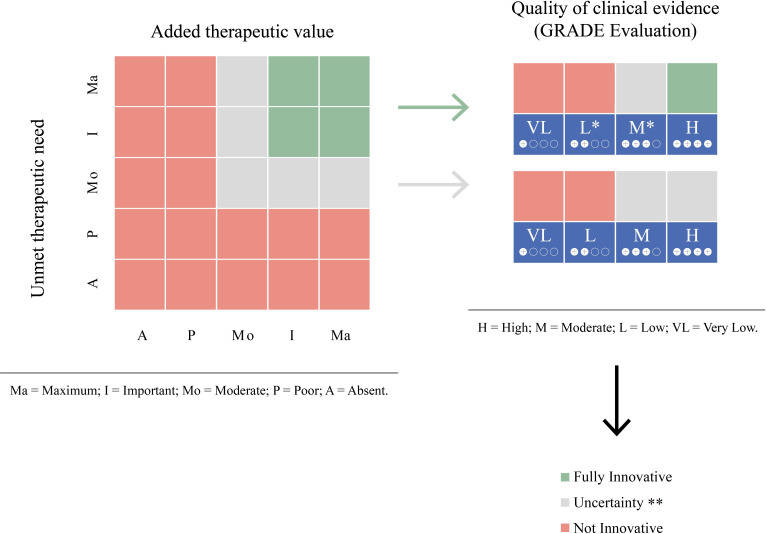
Figure 1.
Criteria used to evaluate innovativeness adopted by the Italian Medicines Agency (adapted from Recchia, 2017).10 *For rare disease there is the following exception: the fully innovative is attributed in the presence of at least important unmet therapeutic need and added therapeutic value in presence of at least low quality of clinical evidence. **The innovativeness appraisal has to be decided on a case-by-case basis. GRADE, Grading of Recommendations Assessment, Development and Evaluation.