Abstract
The developing brain is highly sensitive to the hormonal milieu, with gonadal steroid hormones involved in neurogenesis, neural survival, and brain organization. Limited available evidence suggests that endocrine‐disrupting chemicals (EDCs) may perturb these developmental processes. In this study, we tested the hypothesis that prenatal exposure to a mixture of polychlorinated biphenyls (PCBs), Aroclor 1221, would disrupt the normal timing of neurogenesis in two hypothalamic regions: the ventromedial nucleus (VMN) and the preoptic area (POA). These regions were selected because of their important roles in the control of sociosexual behaviors that are perturbed in adulthood by prenatal EDC exposure. Pregnant Sprague–Dawley rats were exposed to PCBs from Embryonic Day 8 (E8) to E18, encompassing the period of neurogenesis of all hypothalamic neurons. To determine the birth dates of neurons, bromo-2-deoxy-5-uridine (BrdU) was administered to dams on E12, E14, or E16. On the day after birth, male and female pups were perfused, brains immunolabeled for BrdU, and numbers of cells counted. In the VMN, exposure to PCBs significantly advanced the timing of neurogenesis compared to vehicle‐treated pups, without changing the total number of BrdU+ cells. In the POA, PCBs did not change the timing of neurogenesis nor the total number of cells born. This is the first study to show that PCBs can shift the timing of neurogenesis in the hypothalamus, specifically in the VMN but not the POA. This result has implications for functions controlled by the VMN, especially sociosexual behaviors, as well as for sexual selection more generally.
Keywords: Aroclor 1221, endocrine-disrupting chemical, hypothalamus, neurogenesis, polychlorinated biphenyl, preoptic area, ventromedial nucleus
1 |. INTRODUCTION
Polychlorinated biphenyls (PCBs) are a class of endocrine-disrupting chemical (EDC) present in the bodies of humans and wildlife due to widespread environmental contamination (Woodruff, Zota, & Schwartz, 2011). PCBs are known neurotoxicants, and exposure in humans is associated with deficits in cognitive behaviors (language, memory, and reaction time) and increases in affective disorders such as depression and anxiety (Boucher et al., 2012; Boucher, Muckle, & Bastien, 2009; Caspersen et al., 2016; Fitzgerald et al., 2008; Gaum et al., 2017; Newman et al., 2006; Patandin et al., 1999; Plusquellec et al., 2010; Schantz, Widholm, & Rice, 2003; Šovčíková et al., 2015). Widespread contamination of the environment with PCBs places a measurable economic burden on society due to the increase of individuals with intellectual disabilities (Bellanger, Demeneix, Grandjean, Zoeller, & Trasande, 2015). Studies conducted in both laboratory animals and wildlife also show behavioral perturbations, including social and sociosexual behaviors, that could impair reproductive success and sexual selection (Gore, Holley, & Crews, 2018; Gore, Krishnan & Reilly, 2019).
Exposures to PCBs during developmental periods of life when hormonally sensitive neural circuits are becoming organized are particularly important in determining neuroendocrine outcomes later in life. Indeed, early life exposures of rats or mice to PCBs led to increased anxiety-like behaviors and reduced or altered social and sociosexual interactions in adolescents and adults (Bell, Thompson, Rodriguez, & Gore, 2016; Gillette et al., 2017; Jolous-Jamshidi, Cromwell, McFarland, & Meserve, 2010; Karkaba, Soualeh, Soulimani, & Bouayed, 2017; Reilly et al., 2015; Steinberg, Juenger, & Gore, 2007; Topper et al., 2019; Wang, Fang, Nunez, & Clemens, 2002). In a few studies, these behavioral outcomes were associated with changes to gene and/or protein expression measured in the same adolescent or adult animals (Bell et al., 2016; Topper et al., 2019), but the mechanism for how the PCBs might cause these changes is unknown.
Among the brain organizational processes that PCBs and other EDCs may perturb is neurogenesis because of the sensitivity of this process to prenatal hormones, especially estrogens (Kinch, Ibhazehiebo, Jeong, Habibi, & Kurrasch, 2015; Martínez-Cerdeño, Noctor, & Kriegstein, 2006; Okada et al., 2008; Ruiz-Palmero et al., 2016; Scerbo et al., 2014). A very limited number of studies have investigated effects of EDCs (but not PCBs) on brain neurogenesis (Fini et al., 2017; Fritsche, Cline, Nguyen, Scanlan, & Abel, 2005; Kinch et al., 2015; Naveau et al., 2014; Okada et al., 2008; Tofighi et al., 2011). Several studies suggest PCBs promote neural differentiation through actions on the neural stem cell niche, although results have been mixed (Fritsche et al., 2005; Naveau et al., 2014; Tofighi et al., 2011).
Previously, we showed that prenatal exposure to the PCB mixture Aroclor 1221 (A1221) led to sex-specific alterations in hypothalamic gene and protein expression, and adult behavior in rats (Gillette et al., 2017; Reilly et al., 2015; Topper et al., 2019). In the present study, we tested whether exposure to A1221 would affect the timing of neurogenesis in two hypothalamic brain regions, the ventromedial nucleus (VMN) and the preoptic area (POA), involved in the control of sociosexual behavior (Kuroda & Numan, 2014; Oomura, Aou, Koyama, Fujita, & Yoshimatsu, 1988; Robarts & Baum, 2006; Spiteri et al., 2010; Will, Hull, & Dominguez, 2014; Williamson, Romeo, & Curley, 2017; Yang et al., 2013) and regions in which A1221 changes profiles of gene expression (Topper et al., 2019). The laboratory rat is an excellent model for bidirectional translation to humans and wildlife species because of the high conservation of the hypothalamus in all vertebrate species.
2 |. MATERIALS AND METHODS
2.1 |. Experimental design
All animal procedures were conducted in accordance with protocols approved by IACUC at the University of Texas at Austin. Sprague–Dawley rats were purchased from Harlan and Envigo (Houston, TX), and housed in a colony room with controlled temperature (22°C) and light cycle (14:10 dark:light, lights on at 0100). Virgin females were mated with sexually experienced males, and the day after mating was termed Embryonic Day 1 (E1).
Pregnant rat dams were exposed to one of two treatments via daily intraperitoneal injection from E8 to E18 (total 11 injections). The vehicle (6% dimethyl sulfoxide [DMSO] in sesame oil) was given alone or used to deliver 1 mg/kg A1221 (C-221N, Lot: 072-202-01; AccuStandard, New Haven, CT), at a volume of ~0.1 ml. The timing of treatment and dosages was selected to match prior work, to approximate human fetal exposure levels, and to span the period of fetal gonadal development and the early stages of brain sexual differentiation (Krishnan, Hasbum et al., 2019; Krishnan et al., 2018; Krishnan, Rahman et al., 2019; Lackmann, 2002; Lanting et al., 1998). Although we did not measure fetal levels of A1221, we have previously predicted that pups were exposed to ~2 μg/kg at this maternal dose (Steinberg, Walker, Juenger, Woller, & Gore, 2008; Takagi, Aburada, Hashimoto, & Kitaura, 1986). The exposure window also spans the entirety of hypothalamic neurogenesis (Shimada & Nakamura, 1973). To label cells exiting the cell cycle, each dam was also given one intraperitoneal injection of bromo-2-deoxy-5-uridine (BrdU) on either E12, E14, or E16. These ages were chosen to span the peak of neurogenesis in the POA and VMN (Bayer & Altman, 1987; Cheung, Kurrasch, Liang, & Ingraham, 2013; Shimada & Nakamura, 1973). Dams were assigned to treatment groups as shown in Table 1, with n’s indicated for the final number of dams (litters) and individual pups. The day of parturition was termed Postnatal Day 0 (P0). At P1, pups were weighed and their anogenital distance (AGD) was measured. The body weight and AGD were used to calculate an anogenital index (AGI) using the following formula . Up to two male and female pups with median AGIs were selected from each litter (no more than three pups total from one litter). Pups were collected for each group according to Table 1. The total pups per treatment includes males and females combined, as statistical analysis showed no sex difference in results. Due to tissue loss during perfusion, sectioning, or processing, final n’s for analyses (indicated in Figures 2 and 4) were between 10 and 14 per group. The pups were anesthetized with an overdose of ketamine and xylazine and transcardially perfused with normal saline followed by 4% paraformaldehyde (Dickerson, Cunningham, & Gore, 2011). The perfused brains were removed and postfixed in 4% paraformaldehyde at 4°C overnight. Whole brains were cryoprotected in a series of sucrose solutions and cryoprotection buffer containing ethylene glycol, glycerol, and phosphate-buffered saline. Cryoprotected brains were stored at −20°C until sectioned.
TABLE 1. Treatment assignments of dams and litter utilization.
| Treatment | BrdU injection day | N of dams (litters) | N of pups included | Total pups per treatment |
|---|---|---|---|---|
| DMSO | E12 | 6 | 13 | 41 |
| E14 | 6 | 14 | ||
| E16 | 7 | 14 | ||
| PCB | E12 | 8 | 12 | 41 |
| E14 | 7 | 14 | ||
| E16 | 8 | 15 |
Note: Dams receiving DMSO or A1221 were injected with BrdU on E12, E14, or E16. One to three pups from each litter were used for each endpoint. N refers to males and females combined, as there was no sex difference.
Abbreviations: A1221, Aroclor 1221; BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; E12, Embryonic Day 12; PCB, polychlorinated biphenyl.
FIGURE 2.
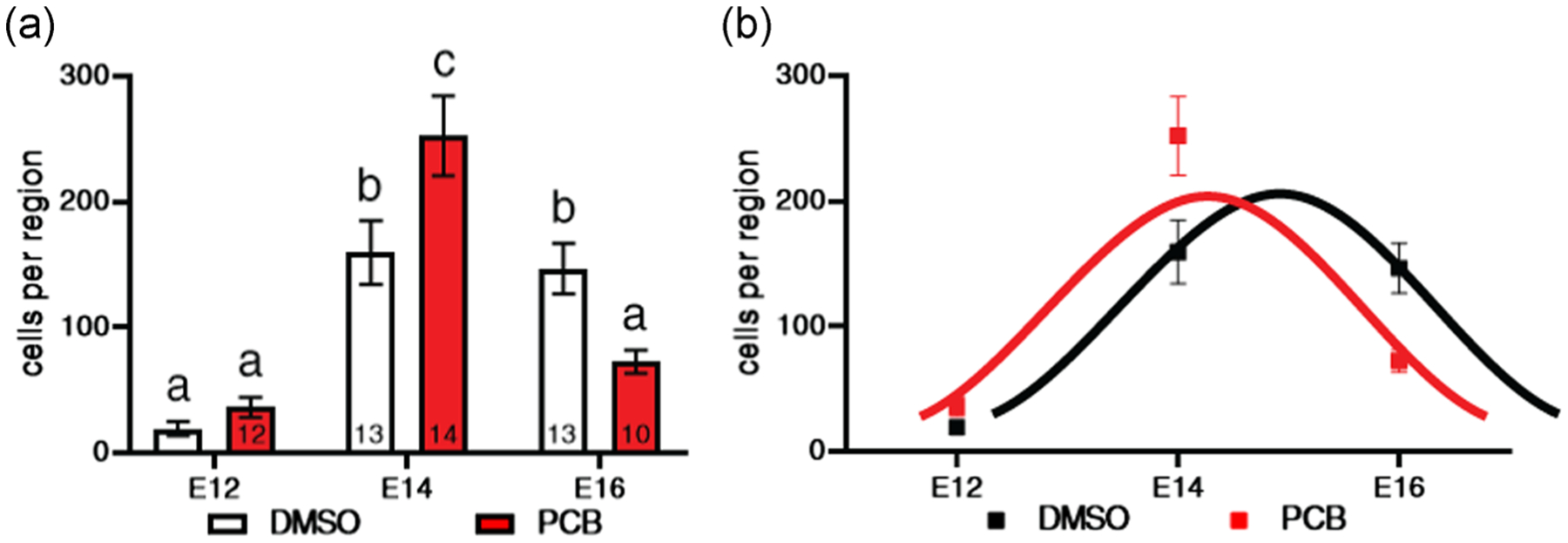
The number of BrdU+ cells in the VMN of P1 pups is shown in relationship to day of injection of the dams with BrdU. (a) Bars with different letters are significantly different. The numbers of pups per group are shown within bars except for E12 DMSO (n = 12). (b) The same data are shown with curves fitted to the timecourse of neurogenesis (black for DMSO and red for PCB). PCB treatment shifted the timing of neurogenesis, with the peak occurring about a day earlier (~E14) than the DMSO group (~E15). BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; E12, Embryonic Day 12; P1, Postnatal Day 1; PCB, polychlorinated biphenyl; VMN, ventromedial nucleus
FIGURE 4.
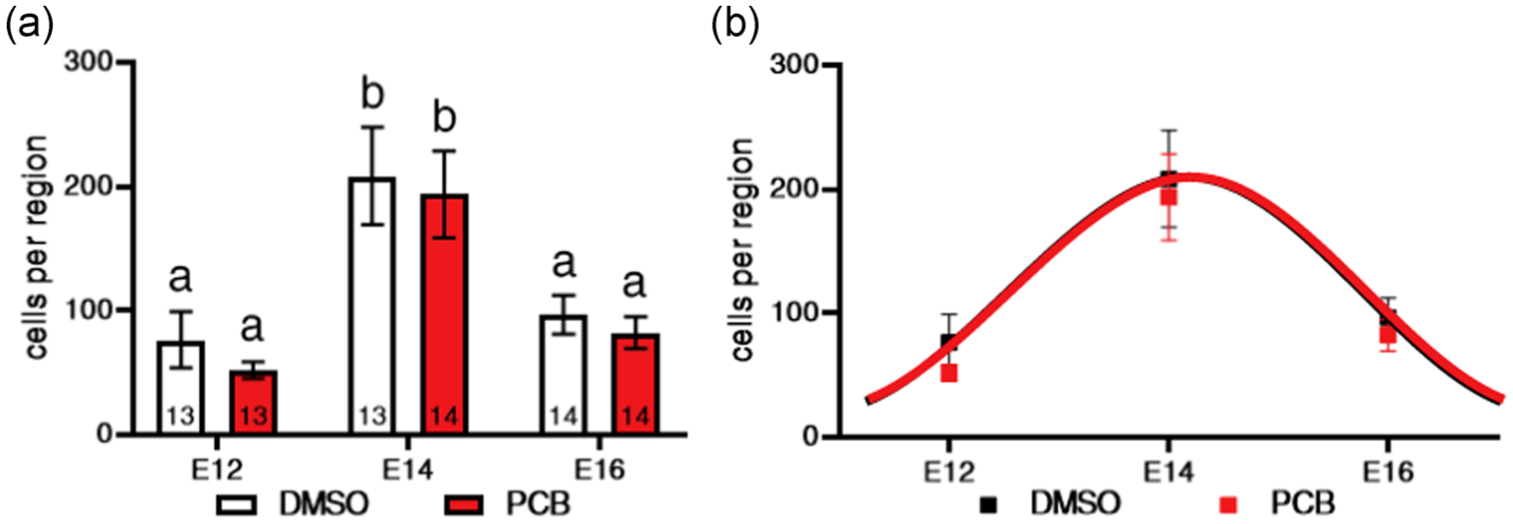
The number of BrdU+ cells in the POA of P1 pups is shown in relationship to day of injection of the dams with BrdU. (a) Bars with different letters are significantly different. Numbers of pups per group are shown within bars. (b) The same data are shown with curves fitted to the timecourse of neurogenesis (black for DMSO and red for PCB). The curves are entirely superimposed, with the peak for both PCB and DMSO occurring at ~E14. BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; E14, Embryonic Day 14; P1, Postnatal Day 1; PCB, polychlorinated biphenyl; POA, preoptic area
2.2 |. Solution preparation
BrdU (B5002; Sigma-Aldrich) was dissolved in 0.007 M NaOH at a concentration of 20 mg/ml. Prepared solutions were stored at −20°C. At the time of injection preparation, solutions were thawed and vortexed at room temperature to redissolve precipitated solute. Pregnant dams were given a single 25 mg/kg injection of BrdU on E12, E14, or E16 (Al-Shamma & De Vries, 1996).
2.3 |. Tissue processing and immunohistochemistry
Brains were embedded in 7% agarose and sectioned on a vibrating microtome (Leica) at a thickness of 40 μm. Every third section was collected to form a 1:3 series (e.g., Sections 1, 4, 7). From one series per rat, three sections containing the POA and VMN were selected for immunohistochemistry. Free-floating sections were incubated in preheated 2 N HCl at 37°C for 30 min. Next, sections were blocked in 10% normal goat serum (Vector) and 0.5% Triton-X (Sigma-Aldrich) for 1 hr at room temperature. Sections were incubated overnight at 4°C with sheep-anti-BrdU (1:1,000) primary antibody (ab1893; Abcam) with 1% normal goat serum and 0.5% Triton-X. Sections were incubated for 2 hr at room temperature in the dark with donkey-anti-sheep Alexa Fluor 488 (1:500) fluorescent secondary antibody (A-11015; Thermo Fisher Scientific) in 1% normal goat serum and 0.5% Triton-X. Sections were mounted and coverslipped with fluorescent mounting media containing 4′,6-diamidino-2-phenylindole (Vector).
2.4 |. Confocal imaging
Images were collected using a Zeiss 710 Laser Scanning Confocal microscope. Ten-micrometer Z-stacks with images collected every 1 μm were taken at ×20 magnification. The field of view constituted a 425 × 425 μm region of interest. This region of interest covers ~70% of the VMN and ~50% of the POA. Z-stacks were collapsed into a projection image and all BrdU+ cells were counted within the frame by an experimenter blinded to the EDC treatment groups and BrdU injection day.
2.5 |. Statistical analysis
T tests within treatments were used to determine whether BrdU labeling differed by sex. Because no main effects of sex were found for the neurogenesis endpoints in VMN or POA, statistical analysis was conducted for the sexes combined. Two-way analysis of variance (ANOVA) was used to examine differences related to treatment and BrdU injection day. Multiple comparisons were used to examine treatment differences on specific days if the main effect of treatment or interaction was identified. p values were adjusted for multiple comparisons using Sidak’s multiple comparisons tests. For sexually dimorphic developmental endpoints (body weight, AGD, and AGI), data were analyzed separately by sex. T tests within sex were used to examine treatment differences. GraphPad Prism version 8.4.3 was used to run T tests, ANOVA, and Sidak’s multiple comparisons test and generate the corresponding figures. Data are presented as mean ± standard error of the mean.
MatLab2018b version 9.5.0.1033004 was used to fit Gaussian distributions to the mean cell count data across E12, E14, and E16 for each treatment group. Estimates were given by the “fit” function from the curve fitting toolbox. For bootstrapping analysis, each replicate resulted in a sample size of 14 to match the desired sampling strategy in the experiment. It was possible for missing data to be included on any day there was an n < 14. In other words, E12 DMSO animals’ datapoints were selected at random, with replacement, 14 times. Then, E14 DMSO data went through the same process, and then E16 DMSO data, until 14 animals were selected for each embryonic day. For each replicate of 14 animals, the mean cell count was determined at each embryonic day. Missing data were disregarded when the means were calculated. A Gaussian distribution was fitted to the replicate using “gauss1” as the “fit type” specification, using the formula (MathWorks, 2020). The three parameters a, b, and c corresponded to the height, peak location, and width/standard deviation of the curve, respectively. After each replicate was produced and fitted, the parameters were stored. This was repeated 10,000 times. The formula was used to calculate the probability of overlap events.
2.6 |. Control of risk of bias
The following procedures were employed to reduce the risk of bias and confounding variables. Treatments were coded so that all experimenters and assistants were blind to PCB and vehicle groups. Virgin female rats were mated and randomly assigned to treatments, and there were no differences in dam age or weight. Dams were assigned to BrdU injection days systematically to ensure that all groups were represented equally throughout the treatment period to avoid a cohort effect. On P1, the pups with the most median AGI of each litter were collected to ensure that the observed characteristics represented the most central tendency of the population. During tissue processing and imaging, each batch contained representatives of all coded groups. Similarly, BrdU counts were performed blind to treatment and BrdU injection day. To reduce the risk of bias during data analysis, a priori hypotheses and statistical tests were established. The VMN and POA as well as BrdU injections days were selected as targets before collecting tissue. Two-way ANOVAs comparing EDC treatment and BrdU injection day with multiple comparisons between BrdU days both within and between treatments were planned analyses. Treatments were decoded after completion of all experimental work for figure and manuscript preparation.
3 |. RESULTS
3.1 |. Developmental measures
There was no effect of treatment on the body weight, AGD, or AGI of male or female pups on P1 (Table 2). While there was no effect of treatment on overall litter size (Table 2), PCB litters had a significantly higher male to female sex ratio than DMSO litters (Welch’s t test, t = 2.914, df = 35.08; p = .0062).
TABLE 2.
Birth outcomes at P1
| DMSO | PCB | |||||
|---|---|---|---|---|---|---|
| Mean | SEM | N | Mean | SEM | N | |
| Males | ||||||
| Body weight (g) | 6.44 | 0.26 | 20 | 6.52 | 0.14 | 19 |
| AGD (mm) | 3.50 | 0.09 | 20 | 3.60 | 0.08 | 19 |
| AGI | 1.88 | 0.03 | 20 | 1.93 | 0.04 | 19 |
| Females | ||||||
| Body weight (g) | 6.08 | 0.22 | 21 | 6.34 | 0.16 | 22 |
| AGD (mm) | 1.73 | 0.05 | 21 | 1.81 | 0.05 | 22 |
| AGI | 0.95 | 0.02 | 21 | 0.98 | 0.02 | 22 |
| Sexes combined N | 41 | 41 | ||||
| Litter size | 12.6 | 0.5 | 19 | 11.6 | 0.7 | 23 |
| Sex ratio (M:F) | 0.87 | 0.16 | 19 | 1.80** | 0.27 | 23 |
Note: There were no treatment differences in body weight, anogenital distance (AGD), or anogenital index (AGI) in male or female P1 pups. Ns for body weight, AGD, and AGI refers to individual pups. The total number of pups for each treatment is indicated for the sexes combined (N = 41 each for DMSO and PCB). DMSO and PCB litters were similar in size, but PCB litters had a significantly higher ratio of male to female pups than DMSO. N for litter size and sex ratio refers to entire litters.
Abbreviations: DMSO, dimethyl sulfoxide; P1, Postnatal Day 1; PCB, polychlorinated biphenyl; F, female; M, male.
p < .01.
3.2 |. PCB exposure shifted the timing of neurogenesis in the VMN
The number of cells exiting the cell cycle (BrdU+) was counted in the VMN of male and female pups from dams (DMSO or PCB treated) given an injection of BrdU on E12, E14, or E16. Because there was no main effect of sex, data from males and females are combined. The region of the VMN that was analyzed (Paxinos, 1991) and a representative fluorescence micrograph of BrdU labeling is shown in Figure 1. Results showed that both the DMSO and PCB groups had a peak in cells exiting the cycle between E14 and E16 (Figure 2), consistent with the peak of neurogenesis in the rat VMN that has been reported to occur at E15 (Altman & Bayer, 1978). A two-way ANOVA revealed a main effect of embryonic day on the number of BrdU+ cells (F(2, 67) = 43.37; p < .0001). In DMSO pups, the number of cells labeled on E14 and E16 was significantly greater than the number labeled on E12 (Figure 2a). There was no difference between E14 and E16. In PCB pups, there were significantly more cells labeled on E14 than both E12 and E16, but E12 and E16 were not different from each other. There was also a significant interaction between treatment and embryonic day (F(2,67) = 10.38; p < .0001). Significantly more cells exited the cell cycle on E14 and significantly fewer cells exited the cell cycle on E16 in PCB pups, resulting in a shift in the developmental curve to about a day earlier (E14 for PCB and E15 for DMSO) but no overall change in cell numbers (Figure 2b).
FIGURE 1.
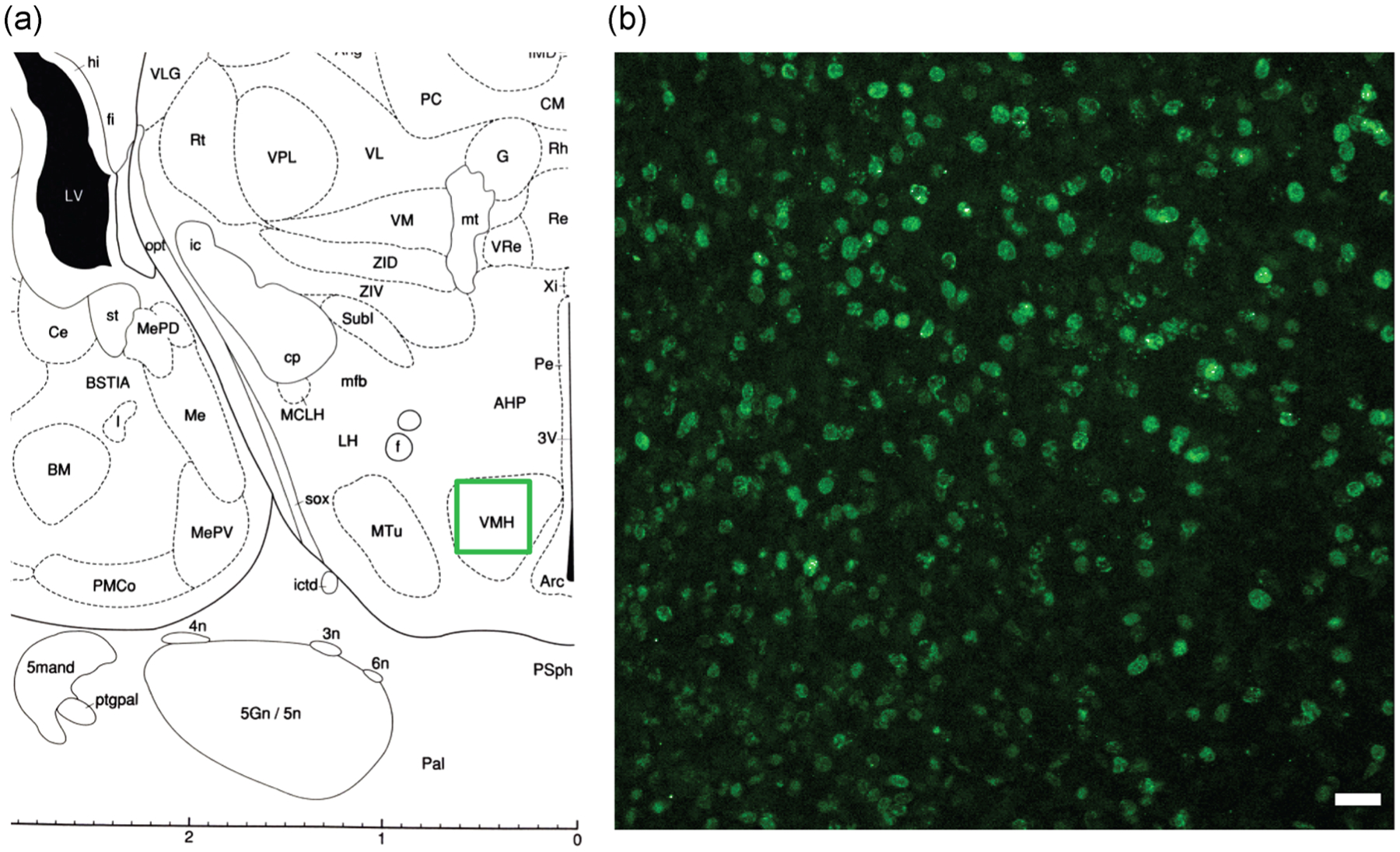
(a) The region of the ventromedial nucleus (labeled here as VMH) used for the analysis is shown, with the region of interest indicated with a green outline. (b) A representative image of BrdU nuclear labeling in the VMN of a DMSO female pup whose dam was injected with BrdU on E14. The region of interest outlined in green (a) is shown at ×20 magnification in (b). White scale bar = 25 μm. The brain map shown in (a) was modified from Paxinos (1991). BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; E14, Embryonic Day 14; VMH, ventromedial nucleus of the hypothalamus; VMN, ventromedial nucleus
3.3 |. PCB exposure did not change the timing of neurogenesis in the POA
A similar analysis was conducted in the POA. Again, there was no effect of sex, so males and females were combined. Figure 3 shows the region of analysis (Paxinos, 1991) and BrdU immunofluorescence in the POA of a representative DMSO pup on E14. Both treatment groups showed a peak in cells exiting the cell cycle between E14 and E16 in the POA, consistent with the peak of neurogenesis reported to occur at E15 in the rat POA (Altman & Bayer, 1978; Bayer & Altman, 1987; Figure 4). A two-way ANOVA revealed a main effect of embryonic age (F(2, 75) = 17.08; p < .0001). In both DMSO and PCB pups, significantly more cells exited the cell cycle on E14 than E12 or E16 and the number of BrdU+ cells on E12 and E16 was not different from one another. There was no effect of treatment nor an interaction of treatment and embryonic day in this region. As shown in Figure 4b, the neurogenesis curves for DMSO and PCB pups were entirely superimposed. There was no difference in the total number of BrdU+ cells measured across the 3 days between groups.
FIGURE 3.

(a) The region of the preoptic area (labeled here as MPO) used for the analysis is shown, with the region of interest indicated with a green outline. (b) A representative image of BrdU nuclear labeling in the POA of a DMSO female pup whose dam was injected with BrdU on E14. The region of interest outlined in green (a) is shown at ×20 magnification in (b). White scale bar = 25 μm. The brain map shown in (a) was modified from Paxinos (1991). BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; E14, Embryonic Day 14; MPO, medial preoptic area; POA, preoptic area
3.4 |. PCB exposure significantly changes the timecourse of neurogenesis in the VMN but not POA
We hypothesized that peak embryonic neurogenesis, or the peak loci, were significantly shifted by PCB treatment in the VMN. To statistically evaluate the difference between peak neurogenesis timing in DMSO and PCB pups, bootstrapping analysis was performed (Figure 5a). Cell count samples were replicated 10,000 times with replacement across E12, E14, and E16. Then, a Gaussian distribution was fitted to the mean cell count of each replicate. The three parameters we fitted were the location of the peak (along the x-axis), the amplitude of the peak (along the y-axis; height), and the width of the curve. For our purposes, this translated into when peak neurogenesis occurred, the number of cells exiting the cell cycle at that time, and the estimated duration of neurogenesis, respectively. From these replicates, we determined the number of instances in which the peak loci of the DMSO and PCB curve overlapped; in other words, how frequently the PCB peak loci were greater than the DMSO peak loci. This occurred zero times in 10,000 replications, indicating that the probability of the PCB peak occurring after the DMSO peak is p ~0; 10,000 bootstrap replications.
FIGURE 5.

Examples of the bootstrapping process in the VMN (a) and POA (b). The fitted curves of 500 bootstrapped replicates (DMSO in gray and PCB in red) against fits to the original dataset (black lines; original means are open circles for DMSO and open squares for PCB). The distributions of peak loci over 10,000 replicates for each group are inset at the bottom; DMSO in white and PCB in red. Ninety-five percent confidence intervals for the distributions are shown as dashed vertical lines. BrdU, bromo-2-deoxy-5-uridine; DMSO, dimethyl sulfoxide; PCB, polychlorinated biphenyl; POA, preoptic area; VMN, ventromedial nucleus
A similar analysis was conducted for the POA (Figure 5b), revealing that there was substantial overlap in the distributions of peak loci. The null hypothesis (DMSO peak loci = PCB peak loci) would result in an even split of replicates (5,000 out of 10,000) in which each group is greater than the other; in other words, whether the PCB peak or DMSO peak occurs first is due entirely to chance p = .5; 10,000 bootstrap replicates. For the POA data, the peak loci of the DMSO and PCB curves overlapped in 6,479 instances. This finding indicates that the probability of the PCB peak occurring after the DMSO peak is p = .6479; 10,000 bootstrap replications. This probability indicates there was not a significant treatment effect on the timing of peak neurogenesis in the POA.
4 |. DISCUSSION
To the best of our knowledge, this study is the first to show that PCBs can advance the timing of neurogenesis in the developing hypothalamus. This finding extends previous research by showing that an EDC can act in a subregion-specific manner within the hypothalamus, with the VMN affected while the POA is not. Disruption to the normal developmental timing of neuron birth in the VMN could lead to inappropriate synapse formation and pruning in later neurodevelopment. While the mechanism by which PCBs act to promote cell cycle exit in this population remains unknown, work on the role of hormones in progenitor cell proliferation, differentiation, and migration suggests that these underlying processes may be targets, and merit future research.
Another study showed that a PCB mixture can promote neurogenesis in the developing rat cortex (Naveau et al., 2014). PCBs and other EDCs also alter the proliferation and survival of neural progenitor cells in vitro and in vivo. Because of the roles of gonadal hormones (estradiol, testosterone) and the potential for testosterone to be aromatized to estradiol in the neonatal brain (Martínez-Cerdeño et al., 2006; Ruiz-Palmero et al., 2016), some studies have compared EDCs to estradiol, and/or blocked estrogen or androgen receptors. These published results, discussed below, have not clarified a common mechanism but suggest that various hormonal mechanisms are involved.
4.1 |. EDCs can act through estrogenic pathways to alter proliferation
Neural progenitor cells are sensitive to estradiol. This hormone induces their proliferation, and treatment with an estrogen receptor α (ERα) antagonist demonstrated that ERα activation is necessary for normal rates of proliferation in vivo (Martínez-Cerdeño et al., 2006). Similarly, locally synthesized estradiol via aromatization is sufficient to enhance proliferation (Martínez-Cerdeño et al., 2006). Cultured cortical cells showed increased proliferation in response to bisphenol A (BPA) exposure in a manner identical to the application of estradiol (Okada et al., 2008), a result consistent with the estrogenic properties of BPA. Low dose BPA increased proliferation in the hippocampus and midbrain, but higher doses had the opposite effect (Liu et al., 2013; Tiwari et al., 2015).
Cortical neural progenitor cells cultured with PCBs had reduced proliferation (Tofighi et al., 2011). While we did not measure proliferating cells specifically, the PCB pups had similar total levels of cells exiting the cell cycle across the 3 days. If PCBs act through estrogenic mechanisms to promote proliferation, there would likely be increased total cells. PCBs acting to reduce proliferation would not explain our findings.
4.2 |. Neurogenesis and neural differentiation are sensitive to estrogen and androgen signaling
Sex differences in neurogenesis suggest a role for sex steroid hormones in regulating this developmental process. Sex differences in the rate of differentiation were noted in E14 mouse embryos; female cells progressed through developmental stages more quickly than male cells, and only male cell development was enhanced by estradiol treatment (Scerbo et al., 2014). Interestingly, estradiol was found to abolish the sex difference in the expression of the neurogenin 3 gene. Knockdown of neurogenin 3 showed that changes to the expression of this single gene were sufficient to increase or decrease the rate of differentiation in these cells. This phenomenon has also been reported in E17 hippocampal cells (Ruiz-Palmero et al., 2016). Hypothalamic neurons cultured from E16 rat embryos had an estradiol-dependent enhancement of axon growth, and cells from male embryos had a much larger response to estradiol than those from females (Carrer, Cambiasso, & Gorosito, 2005). To our knowledge, a sex difference in differentiation in response to EDC treatment has not been reported, and we did not find a sex difference in the effect of PCB exposure on the timing of neurogenesis in this study.
EDCs can alter the timing of cell cycle exit and the pattern of differentiation in neural cells. PCBs 153 and 180 promoted differentiation (neurite formation) in cultured cortical neural stem cells (Tofighi et al., 2011). A1254, a PCB mix similar to the one used in this study, when administered from E6-21 induced cell cycle exit only on E17 in the cortex of rat embryos (Naveau et al., 2014). Our study was the first to demonstrate that estrogenic PCBs (A1221) could influence the timing of neurogenesis in the hypothalamus in vivo. In the VMN of our PCB-exposed pups, the timing of neurogenesis was shifted significantly earlier. That this was region-specific was illustrated by our results showing no such shift in the POA.
Like PCBs, BPA exposure is also associated with an advance in the timing of neurogenesis. Two studies of E14.5 mice exposed to BPA showed increased cell cycle exit between 24- and 48-hr timeframes (Komada et al., 2012; Nakamura et al., 2006). Both BPA and the replacement chemical BPS promoted precocious cell cycle exit specifically in the hypothalamus of zebrafish embryos (Kinch et al., 2015). Despite the body of evidence discussed here and elsewhere that BPA acts through an estrogenic mechanism to influence neural progenitor cells, Kinch et al. (2015) meticulously unraveled an androgen receptor-dependent mechanism involving the upregulation of aromatase and, therefore, local estradiol synthesis. This finding merits further study, and would be interesting in the context of PCBs.
Treatment with the androgen nandrolone favored differentiation of precursors into neurons rather than glia (Brännvall, Bogdanovic, Korhonen, & Lindholm, 2005). Male hippocampal neurons treated with androgens had increased neurogenin 3 expression and enhanced morphological development (Ruiz-Palmero et al., 2016). The female cells in these experiments, however, had reduced neurogenin 3 expressions and stunted morphological development in response to treatment with androgens. While the underlying mechanism altering the timing of cell cycle exit in our study is not known, both estrogen- and androgen-sensitive processes are emerging as targets for further investigation.
4.3 |. PCBs can disrupt the balance of neural cell birth and death
The development and organization of the hypothalamus and other brain regions depend not only on cell differentiation and subsequent neurogenesis but also on processes involved in pruning neurons and synapses in an activity-dependent manner. Programmed cell death, apoptosis, is a necessary part of this balance (Mosley et al., 2017), and apoptosis is both hormone-sensitive (Forger, 2009) and disrupted by EDCs. Previously, our lab showed that A1221 given at two prenatal ages (E16 and E18, in contrast to the current study in which A1221 was given from E8-18) increased apoptosis in the anteroventral periventricular nucleus of the hypothalamus of females but not males (Dickerson et al., 2011). This effect was not seen in the medial preoptic nucleus, a result consistent with the lack of effect of PCBs on neurogenesis shown here. Other studies have shown that PCBs increase cell death in in vitro primary hippocampal but not cortical cultures (Howard, Fitzpatrick, Pessah, Kostyniak, & Lein, 2003). For bisphenols, exposure of larvae to bisphenol F increased apoptosis in the whole brain (Gu et al., 2020), and Xenopus laevis embryos exposed to BPA had increased brain apoptosis (Oka et al., 2003). As a whole, this literature suggests a loss of certain neural populations due to embryonic exposure to EDCs.
While the present study showed that the overall number of BrdU + neurons in the VMN at P1 was unchanged, whereas the developmental neurogenesis curve was shifted leftward, the imbalance in apoptosis, neurogenesis, and other processes involved in neurodevelopment at the appropriate life stages likely results in structural and functional outcomes. This is consistent with changes in hypothalamic gene expression across the life cycle (Dickerson, Cunningham, Patisaul, Woller, & Gore, 2011; Dickerson et al., 2011; Walker, Goetz, & Gore, 2014). Genomic effects of nuclear ERα activation are associated with the developmental regulation of apoptosis (McCarthy, 2008). While PCBs are known to interact with ERα at low doses, we cannot assert that the effects observed in this study were due changes in transcription induced by ERα without directly measuring downstream targets (Bonefeld-Jorgensen, Andersen, Rasmussen, & Vinggaard, 2001). It is likely that multiple developmental processes are interrupted by PCB exposure and are associated with disruptions of behaviors including those regulated by VMN (Topper et al., 2019). As a whole, this study provides a potential mechanism by which environmental EDCs may impair processes involved in reproductive behaviors, the normal expression of which is necessary for reproductive success.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Lawrence Cormack for guidance with the bootstrap statistics. They would also like to thank Amy Weinberg, Mandee Bell, Lindsay Thompson, and Melissa Kang for husbandry and technical assistance. This study was supported by NIH RO1 ES029464 (ACG), T32 DA018926 (MEHS) and Robert Wood Johnson Foundation Health Policy Research Scholars Program.
Funding information National Institute of Environmental Health Sciences, Grant/Award Number: RO1 ES029464; NIDA:, Grant/Award Number: T32 DA018926; Robert Wood Johnson Foundation Health Policy Research Scholars Program
Footnotes
DATA AVAILABILITY STATEMENT
The authors will provide data upon request.
REFERENCES
- Al-Shamma HA, & De Vries GJ (1996). Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. Journal of Neurobiology, 29(1), 91–98. [DOI] [PubMed] [Google Scholar]
- Altman J, & Bayer SA (1978). Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. The Journal of Comparative Neurology, 182(5), 945–971. 10.1002/cne.901820511 [DOI] [PubMed] [Google Scholar]
- Bayer SA, & Altman J (1987). Development of the preoptic area: Time and site of origin, migratory routes, and settling patterns of its neurons. Journal of Comparative Neurology, 265(1), 65–95. 10.1002/cne.902650106 [DOI] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, & Gore AC (2016). Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Hormones and Behavior, 78, 168–177. 10.1016/j.yhbeh.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanger M, Demeneix B, Grandjean P, Zoeller RT, & Trasande L (2015). Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. Journal of Clinical Endocrinology and Metabolism, 100(4), 1256–1266. 10.1210/jc.2014-4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, & Vinggaard AM (2001). Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology, 158(3), 141–153. 10.1016/S0300-483X(00)00368-1 [DOI] [PubMed] [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly É, … Jacobson JL (2012). Response inhibition and error monitoring during a visual Go/No-Go task in Inuit children exposed to lead, polychlorinated biphenyls, and methylmercury. Environmental Health Perspectives, 120, 608–615. 10.1289/ehp.1103828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Muckle G, & Bastien CH (2009). Prenatal exposure to polychlorinated biphenyls: A neuropsychologic analysis. Environmental Health Perspectives, 117, 7–16. 10.1289/ehp.11294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brännvall K, Bogdanovic N, Korhonen L, & Lindholm D (2005). 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. European Journal of Neuroscience, 21(4), 871–878. 10.1111/j.1460-9568.2005.03942.x [DOI] [PubMed] [Google Scholar]
- Carrer HF, Cambiasso MJ, & Gorosito S (2005). Effects of estrogen on neuronal growth and differentiation. Journal of Steroid Biochemistry & Molecular Biology, 93, 319–323. 10.1016/j.jsbmb.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Caspersen IH, Aase H, Biele G, Brantsæter AL, Haugen M, Kvalem HE, … Knutsen HK (2016). The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environment International, 94, 649–660. 10.1016/j.envint.2016.06.033 [DOI] [PubMed] [Google Scholar]
- Cheung CC, Kurrasch DM, Liang JK, & Ingraham HA (2013). Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. Journal of Comparative Neurology, 521(6), 1268–1288. 10.1002/cne.23226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, & Gore AC (2011). Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicology and Applied Pharmacology, 252(1), 36–46. 10.1016/j.taap.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, & Gore AC (2011). Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology, 152(2), 581–594. 10.1210/en.2010-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini J-B, Mughal BB, Le Mével S, Leemans M, Lettmann M, Spirhanzlova P, … Demeneix BA (2017). Human amniotic fluid contaminants alter thyroid hormone signalling and early brain development in Xenopus embryos. Scientific Reports, 7, 43786 10.1038/SREP43786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, … Hicka HE (2008). Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environmental Health Perspectives, 116(2), 209–215. 10.1289/ehp.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG (2009). Control of cell number in the sexually dimorphic brain and spinal cord. Journal of Neuroendocrinology, 21, 393–399. 10.1111/j.1365-2826.2009.01825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E, Cline JE, Nguyen NH, Scanlan TS, & Abel J (2005). Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: Clue for involvement of thyroid hormone receptors. Environmental Health Perspectives, 113(7), 871–876. 10.1289/ehp.7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaum PM, Gube M, Schettgen T, Putschögl FM, Kraus T, Fimm B, & Lang J (2017). Polychlorinated biphenyls and depression: Cross-sectional and longitudinal investigation of a dopamine-related Neurochemical path in the German HELPcB surveillance program. Environmental Health, 16, 106 10.1186/s12940-017-0316-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, & Gore AC (2017). Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Hormones and Behavior, 87, 8–15. 10.1016/j.yhbeh.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Holley AM, & Crews D (2018). Mate choice, sexual selection, and endocrine-disrupting chemicals. Hormones and Behavior, 101, 3–12. 10.1016/j.yhbeh.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Krishnan K, & Reilly MP (2019). Endocrine-disrupting chemicals: Effects on neuroendocrine systems and the neurobiology of social behavior. Hormones and Behavior, 111, 7–22. 10.1016/j.yhbeh.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Wu J, Xu S, Zhang L, Fan D, Shi L, … Ji G (2020). Bisphenol F exposure impairs neurodevelopment in zebrafish larvae (Danio rerio). Ecotoxicology and Environmental Safety, 188, 109870 10.1016/j.ecoenv.2019.109870 [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, & Lein PJ (2003). Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicology and Applied Pharmacology, 190(1), 72–86. 10.1016/S0041-008X(03)00156-X [DOI] [PubMed] [Google Scholar]
- Jolous-Jamshidi B, Cromwell HC, McFarland AM, & Meserve LA (2010). Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicology Letters, 199, 136–43. 10.1016/j.toxlet.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkaba A, Soualeh N, Soulimani R, & Bouayed J (2017). Perinatal effects of exposure to PCBs on social preferences in young adult and middle-aged offspring mice. Hormones and Behavior, 96, 137–146. 10.1016/j.yhbeh.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong J-H, Habibi HR, & Kurrasch DM (2015). Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 112(5), 1475–1480. 10.1073/pnas.1417731112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Asai Y, Morii M, Matsuki M, Sato M, & Nagao T (2012). Maternal bisphenol A oral dosing relates to the acceleration of neurogenesis in the developing neocortex of mouse fetuses. Toxicology, 295(1–3), 31–38. 10.1016/j.tox.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Krishnan K, Hasbum A, Morales D, Thompson LM, Crews D, & Gore AC (2019). Endocrine-disrupting chemicals alter the neuromolecular phenotype in F2 generation adult male rats. Physiology & Behavior, 211, 112674 10.1016/j.physbeh.2019.112674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, & Gore AC (2018). Effects of the endocrine-disrupting chemicals, vinclozolin and polychlorinated biphenyls, on physiological and sociosexual phenotypes in F2 generation sprague-dawley rats. Environmental Health Perspectives, 126(9), 97005 10.1289/EHP3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Rahman S, Hasbum A, Morales D, Thompson LM, Crews D, & Gore AC (2019). Maternal care modulates transgenerational effects of endocrine-disrupting chemicals on offspring pup vocalizations and adult behaviors. Hormones and Behavior, 107, 96–109. 10.1016/j.yhbeh.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda KO, & Numan M (2014). The medial preoptic area and the regulation of parental behavior. Neuroscience Bulletin, 30(5), 863–865. 10.1007/s12264-014-1462-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackmann GM (2002). Polychlorinated biphenyls and hexachlorobenzene in full-term neonates. Neonatology, 81(2), 82–85. 10.1159/000047188 [DOI] [PubMed] [Google Scholar]
- Lanting CI, Huisman M, Muskiet FAJ, Van Der Paauw CG, Essed CE, & Boersma ER (1998). Polychlorinated biphenyls in adipose tissue, liver, and brain from nine stillborns of varying gestational ages. Pediatric Research, 44(2), 222–225. 10.1203/00006450-199808000-00014 [DOI] [PubMed] [Google Scholar]
- Liu R, Xing L, Kong D, Jiang J, Shang L, & Hao W (2013). Bisphenol A inhibits proliferation and induces apoptosis in micromass cultures of rat embryonic midbrain cells through the JNK, CREB and p53 signaling pathways. Food and Chemical Toxicology, 52, 76–82. 10.1016/j.fct.2012.10.033 [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, & Kriegstein AR (2006). Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. European Journal of Neuroscience, 24(12), 3475–3488. 10.1111/j.1460-9568.2006.05239.x [DOI] [PubMed] [Google Scholar]
- MathWorks. (2020). List of Library Models for Curve and Surface Fitting. Retrieved from http://ch.mathworks.com/help/curvefit/list-of-library-models-for-curve-and-surface-fitting.html
- McCarthy MM (2008). Estradiol and the developing brain. Physiological Reviews, 88, 91–124. 10.1152/physrev.00010.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley M, Shah C, Morse KA, Miloro SA, Holmes MM, Ahern TH, & Forger NG (2017). Patterns of cell death in the perinatal mouse forebrain. Journal of Comparative Neurology, 525(1), 47–64. 10.1002/cne.24041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, & Fushiki S (2006). Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A. Journal of Neuroscience Research, 84(6), 1197–1205. 10.1002/jnr.21020 [DOI] [PubMed] [Google Scholar]
- Naveau E, Pinson A, Gerard A, Nguyen L, Charlier C, Thome JP, … Parent AS (2014). Alteration of rat fetal cerebral cortex development after prenatal exposure to polychlorinated biphenyls. PLoS One, 9(3), 1–12. 10.1371/journal.pone.0091903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Aucompaugh AG, Schell LM, Denham M, DeCaprio AP, Gallo MV, … Worswick P (2006). PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicology and Teratology, 28, 439–45. 10.1016/j.ntt.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Oka T, Adati N, Shinkai T, Sakuma K, Nishimura T, & Kurose K (2003). Bisphenol A induces apoptosis in central neural cells during early development of Xenopus laevis. Biochemical and Biophysical Research Communications, 312(4), 877–882. 10.1016/j.bbrc.2003.10.199 [DOI] [PubMed] [Google Scholar]
- Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, & Furukawa Y (2008). Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomedical Research, 29(3), 163–170. 10.2220/biomedres.29.163 [DOI] [PubMed] [Google Scholar]
- Oomura Y, Aou S, Koyama Y, Fujita I, & Yoshimatsu H (1988). Central control of sexual behavior. Brain Research Bulletin, 20(6), 863–870. 10.1016/0361-9230(88)90103-7 [DOI] [PubMed] [Google Scholar]
- Patandin S, Lanting CI, Mulder PGH, Boersma ER, Sauer PJJ, & Weisglas-Kuperus N (1999). Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. Journal of Pediatrics, 134, 33–41. 10.1016/S0022-3476(99)70369-0 [DOI] [PubMed] [Google Scholar]
- Paxinos G (1991). Atlas of the developing rat brain (4th ed). San Diego, CA: Academic Press, Inc. [Google Scholar]
- Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, … Poitras K (2010). The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology, 31(1), 17–25. 10.1016/j.neuro.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, & Gore AC (2015). The effects of prenatal PCBs on adult social behavior in rats. Hormones and Behavior, 73, 47–55. 10.1016/j.yhbeh.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts DW, & Baum MJ (2006). Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Hormones and Behavior, 51, 104–113. 10.1016/j.yhbeh.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palmero I, Ortiz-Rodriguez A, Melcangi RC, Caruso D, Garcia-Segura LM, Rune GM, & Arevalo M-A (2016). Oestradiol synthesized by female neurons generates sex differences in neuritogenesis. Scientific Reports, 6, 31891 10.1038/srep31891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo MJ, Freire-Regatillo A, Cisternas CD, Brunotto M, Arevalo MA, Garcia-Segura LM, & Cambiasso MJ (2014). Neurogenin 3 mediates sex chromosome effects on the generation of sex differences in hypothalamic neuronal development. Frontiers in Cellular Neuroscience, 8, 188 10.3389/fncel.2014.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, & Rice DC (2003). Effects of PCB exposure on neuropsychological function in children. Environmental Health Perspectives, 111, 357–576. 10.1289/ehp.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, & Nakamura T (1973). Time of neuron origin in mouse hypothalamic nuclei. Experimental Neurology, 41(1), 163–173. 10.1016/0014-4886(73)90187-8 [DOI] [PubMed] [Google Scholar]
- Šovčíková E, Wimmerová S, Strémy M, Kotianová J, Loffredo CA, Murínová ĽP, … Trnovec T (2015). Simple reaction time in 8-9-year old children environmentally exposed to PCBs. Neurotoxicology, 51, 138–44. 10.1016/j.neuro.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, & Ågmo A (2010). Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor?? In the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology, 91(2), 142–154. 10.1159/000255766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, & Gore AC (2007). The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Hormones and Behavior, 51(3), 364–372. 10.1016/j.yhbeh.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, & Gore AC (2008). Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: Development, reproductive physiology, and second generational effects. Biology of Reproduction, 78(6), 1091–1101. 10.1095/biolreprod.107.067249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, & Kitaura T (1986). Transfer and distribution of accumulated (14C)polychlorinated biphenyls from maternal to fetal and suckling rats. Archives of Environmental Contamination and Toxicology, 15(6), 709–715. 10.1007/BF01054917 [DOI] [PubMed] [Google Scholar]
- Tiwari SK, Agarwal S, Seth B, Yadav A, Ray RS, Mishra VN, & Chaturvedi RK (2015). Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-catenin pathway. Molecular Neurobiology, 52(3), 1735–1757. 10.1007/s12035-014-8940-1 [DOI] [PubMed] [Google Scholar]
- Tofighi R, Wan Ibrahim WN, Rebellato P, Andersson PL, Uhlén P, & Ceccatelli S (2011). Non-dioxin-like polychlorinated biphenyls interfere with neuronal differentiation of embryonic neural stem cells. Toxicological Sciences, 124(1), 192–201. 10.1093/toxsci/kfr221 [DOI] [PubMed] [Google Scholar]
- Topper VY, Reilly MP, Wagner LM, Thompson LM, Gillette R, Crews D, & Gore AC (2019). Social and neuromolecular phenotypes are programmed by prenatal exposures to endocrine-disrupting chemicals. Molecular and Cellular Endocrinology, 479, 133–146. 10.1016/j.mce.2018.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, & Gore AC (2014). Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal polychlorinated biphenyls in rats. Molecular Endocrinology, 28(1), 99–115. 10.1210/me.2013-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XQ, Fang J, Nunez AA, & Clemens LG (2002). Developmental exposure to polychlorinated biphenyls affects sexual behavior of rats. Physiology and Behavior, 75, 689–96. 10.1016/S0031-9384(02)00673-X [DOI] [PubMed] [Google Scholar]
- Will RG, Hull EM, & Dominguez JM (2014). Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacology, Biochemistry and Behavior, 121, 115–123. 10.1016/j.pbb.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Williamson CM, Romeo RD, & Curley JP (2017). Dynamic changes in social dominance and mPOA GnRH expression in male mice following social opportunity. Hormones and Behavior, 87, 80–88. 10.1016/j.yhbeh.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, & Schwartz JM (2011). Environmental chemicals in pregnant women in the united states: NHANES 2003-2004. Environmental Health Perspectives, 119(6), 878–885. 10.1289/ehp.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, … Shah NM (2013). Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell, 153(4), 896–909. 10.1016/j.cell.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]


