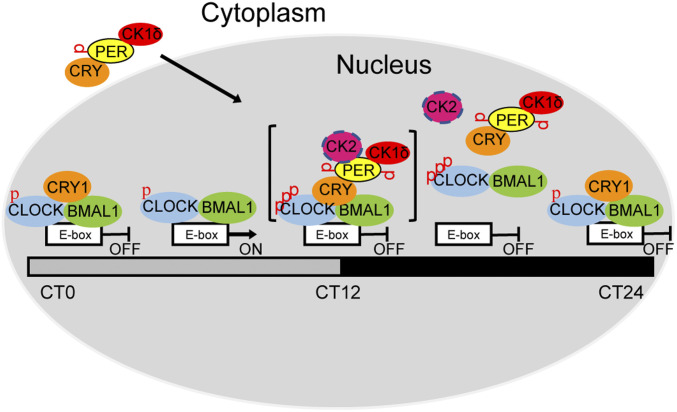
Fig. 7.
Model showing the role of blocking-type and displacement-type repression in the mammalian circadian clock. At around CT4–8, CLOCK–BMAL1 binds to E-boxes to drive clock-controlled gene transcription. After protein synthesis in the cytoplasm, CRY recruits PER and PER recruits CK1δ/CK2 through its CKBD and then enters the nucleus and phosphorylates CLOCK, leading to CLOCK–BMAL1 dissociation from the E-box (CT12–22 displacement-type repression). At around CT0–4, PER levels are too low to be detected and only CRY1 binds to the CLOCK–BMAL1–E-box to block CLOCK–BMAL1 activity (blocking-type repression), which maintains CLOCK–BMAL1 in a repressed state until the next TTFL cycle begins. Note the CK2 is shown a circle with discontinuous circumference to indicate its partial contribution relative to CK1δ. In addition, the entire “repressor–activator complex” is shown in brackets to indicate that it must exist as a kinetic intermediate and not a stable megadalton complex.