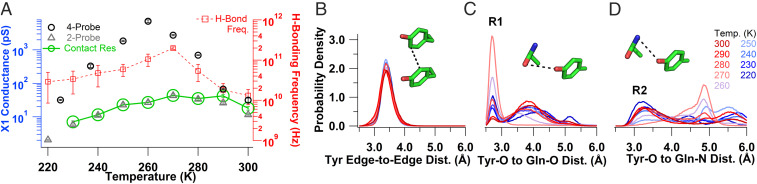
Fig. 6.
Exponentially increasing crystal conductivity upon cooling near physiological temperatures due to increased availability of proton acceptor. (A) Temperature dependence of the X1 conductance measured with a four-probe (black circles) and two-probe (gray triangles) arrangement. The green circles and solid line indicate the inverse of the contact resistance. Errors shown are fit errors to the linear region of an I–V curve measured as in Fig. 2 at each temperature. The calculated hydrogen bonding frequency between Tyr–O and Gln–O is also shown (red squares) with the SEM reported for each temperature (n = 6). (B–D) MD simulations from 300 to 200 K to determine changes in X1 structure upon cooling. (B) Tyrosine edge-to-edge distance. (C) Tyrosine hydroxyl–oxygen to glutamine amide–oxygen distance with the labeled R1 conformation. (D) Tyrosine hydroxyl–oxygen to glutamine amide–nitrogen distances with the labeled R2 conformation. (Insets) The corresponding distances are indicated with a black dotted line with the residues colored as in Fig. 1.